Keywords
carbanions
nitro group
photodynamic therapy (PDT)
photosensitizers
porphyrin complexes
vicarious nucleophilic substitution
β-derivatization
Abstract
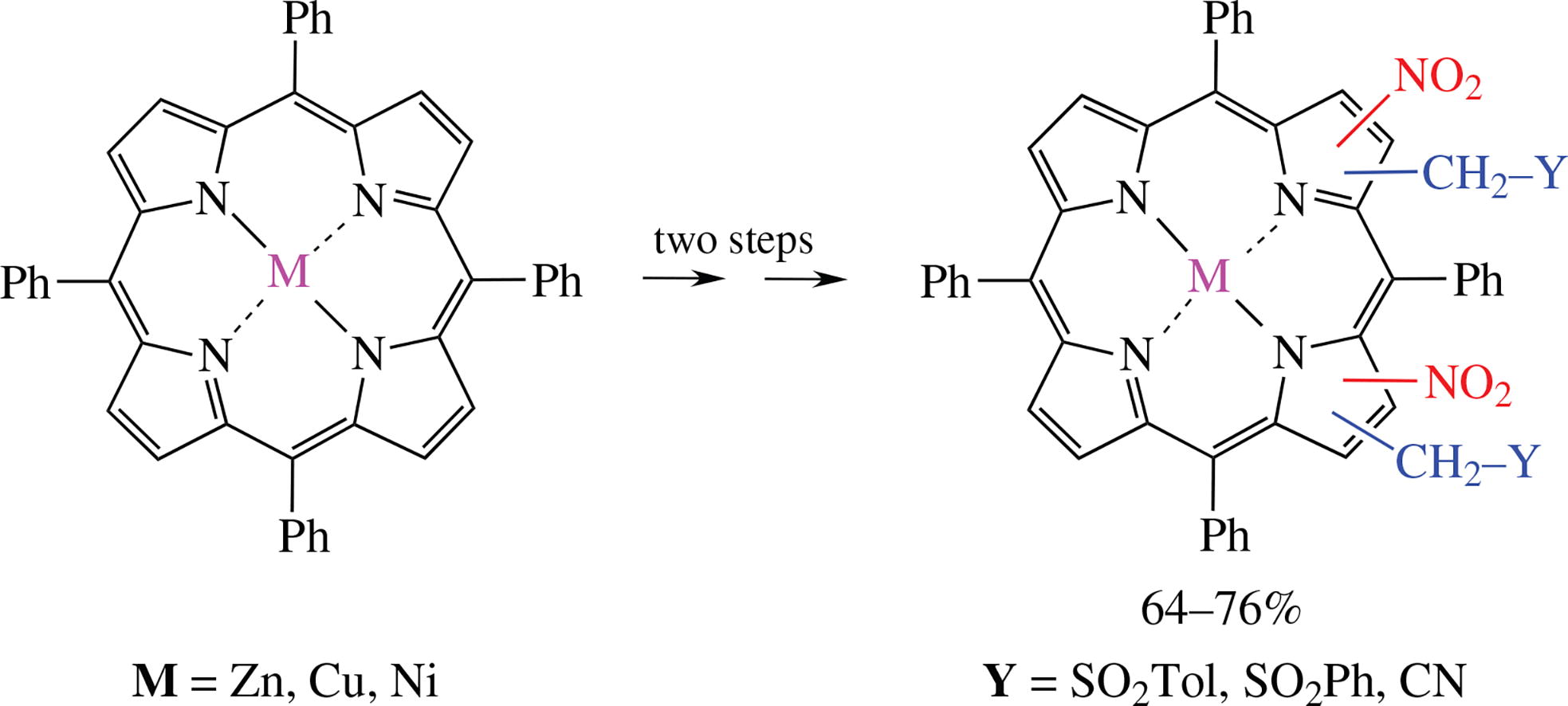
The two-step synthesis of porphyrin derivatives, exhaustively bbb-substituted at the ‘Eastern half’, starting from meso- tetraarylporphyrin chelates (Cuii, Znii, Niii) upon their reaction with nitric acid, is described. The intermediates obtained (mainly dinitro-derivatives) in the reaction with carbanions bearing a leaving group X at the reactive centre give tetrasubstituted at the bbb-positions products, the ones of vicarious nucleophilic substitution of hydrogen. The products seem to be potential anticancer PDT agents, practically unavailable by other methods.
References
1.

Mąkosza M.
Chemical Society Reviews,
2010
2.

Lindsey J.S., Schreiman I.C., Hsu H.C., Kearney P.C., Marguerettaz A.M.
Journal of Organic Chemistry,
1987
3.

Hiroto S., Miyake Y., Shinokubo H.
Chemical Reviews,
2016
4.

Kou J., Dou D., Yang L.
Oncotarget,
2017
5.

Correia J.H., Rodrigues J.A., Pimenta S., Dong T., Yang Z.
Pharmaceutics,
2021
6.

Jacobi P.A., Lanz S., Ghosh I., Leung S.H., Löwer F., Pippin D.
Organic Letters,
2001
7.

Mikus A., Rosa M., Ostrowski S.
Molecules,
2019
8.

Rosa M., Ostrowski S.
ChemistrySelect,
2022
9.

Senge M.O., Sergeeva N.N., Hale K.J.
Chemical Society Reviews,
2021
10.

M\kakosza M., Glinka T., Ostrowski S., Rykowski A.
Chemistry Letters,
1987
11.

Mąkosza M., Ostrowski S.
Journal of the Chemical Society Perkin Transactions 2,
1991
12.

Mikus A., Ostrowski S.
Structural Chemistry,
2022
13.

Mikus A., Ostrowski S.
Structural Chemistry,
2022
14.

Woodward R.B., Ayer W.A., Beaton J.M., Bickelhaupt F., Bonnett R., Buchschacher P., Closs G.L., Dutler H., Hannah J., Hauck F.P., Itǒ S., Langemann A., Le Goff E., Leimgruber W., Lwowski W., et. al.
Tetrahedron,
1990
15.

Mákosza M., Goliński J., Rykowski A.
Tetrahedron Letters,
1983
16.

M?kosza M., Ostrowski S.
Journal für praktische Chemie,
1988
17.

Makosza M., Golinski J., Ostrowski S., Sahasrabudhe A.B., Rykowski A.
Chemische Berichte,
1991
18.

Mikus A., Bielińska M.E., Lipińska T., Ostrowski S.
Synthetic Communications,
2011
19.

Tang M., Liang Y., Lu X., Miao X., Jiang L., Liu J., Bian L., Wang S., Wu L., Liu Z.
Chem,
2021
20.

Murphy R.A., Cava M.P.
Tetrahedron Letters,
1984
21.
Kadish K.M., Smith K.M., Guilard R.
2014