Keywords
cancer cells
nitromethylarenes
o-diarylisoxazoles
sea urchin embryo
X-ray.
Abstract
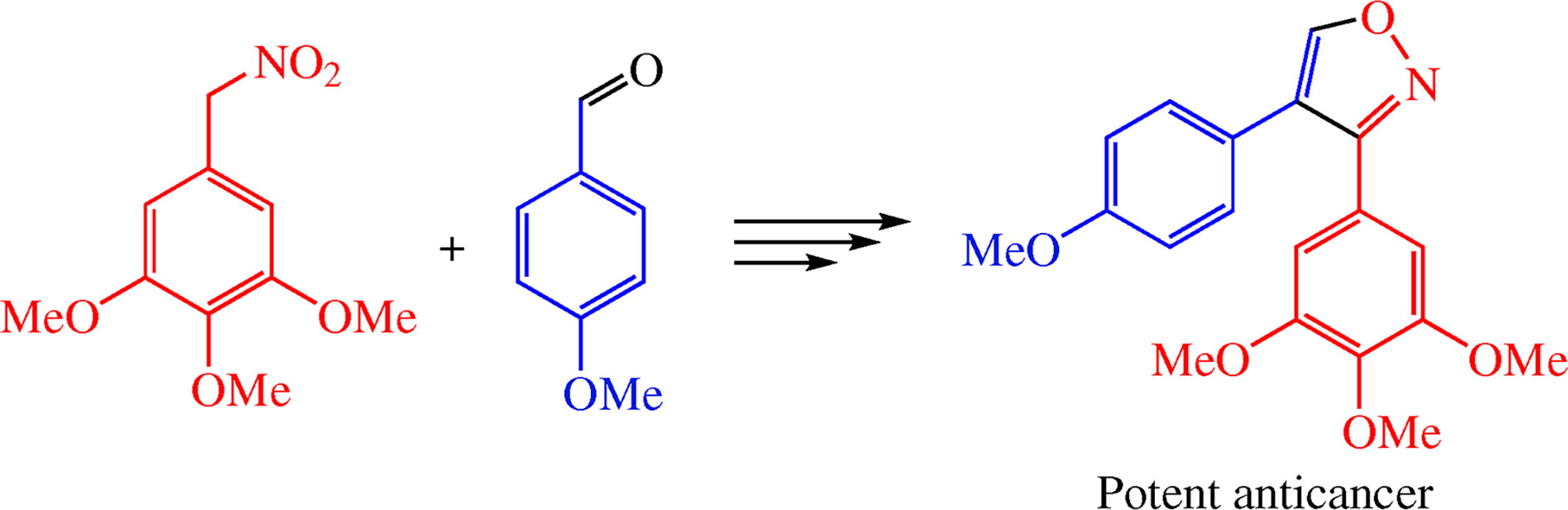
The three-step synthesis of potent anticancer 4-(4-methoxy- phenyl)-3-(3,4,5-trimethoxyphenyl)isoxazole starting from 1,2,3-trimethoxy-5-(nitromethyl)benzene and anisaldehyde was developed. In phenotypic sea urchin embryo assay, this compound exhibited antimitotic antitubulin activity comparable to that of the natural cytostatic combretastatin A4 (CA4). The title isoxazole inhibited in vitro growth of A549 human lung cancer cells and PC-3 human prostate cancer cells with IC50 values of 8 and 6 nm, respectively, exceeding the effect of CA4.
References
.
Silyanova E.A., Ushkarov V.I., Samet A.V., Maksimenko A.S., Koblov I.A., Kislyi V.P., Semenova M.N., Semenov V.V.
Mendeleev Communications,
2022
.

Xue N., Yang X., Wu R., Chen J., He Q., Yang B., Lu X., Hu Y.
Bioorganic and Medicinal Chemistry,
2008
.
Tsyganov D.V., Semenova M.N., Konyushkin L.D., Ushkarov V.I., Raihstat M.M., Semenov V.V.
Mendeleev Communications,
2019
.

Semenova M.N., Demchuk D.V., Tsyganov D.V., Chernysheva N.B., Samet A.V., Silyanova E.A., Kislyi V.P., Maksimenko A.S., Varakutin A.E., Konyushkin L.D., Raihstat M.M., Kiselyov A.S., Semenov V.V.
ACS Combinatorial Science,
2018
.

Kaffy J., Pontikis R., Carrez D., Croisy A., Monneret C., Florent J.
Bioorganic and Medicinal Chemistry,
2006
.

Duan J., Cai X., Meng F., Lan L., Hart C., Matteucci M.
Journal of Medicinal Chemistry,
2007
.

Zhang Q., Peng Y., Wang X.I., Keenan S.M., Arora S., Welsh W.J.
Journal of Medicinal Chemistry,
2007
.

Romagnoli R., Baraldi P.G., Salvador M.K., Preti D., Aghazadeh Tabrizi M., Brancale A., Fu X., Li J., Zhang S., Hamel E., Bortolozzi R., Basso G., Viola G.
Journal of Medicinal Chemistry,
2011
.

Kislyi V.P., Maksimenko A.S., Buikin P.A., Daeva E.D., Semenov V.V.
Synthesis,
2022
.

Jaroch K., Karolak M., Górski P., Jaroch A., Krajewski A., Ilnicka A., Sloderbach A., Stefański T., Sobiak S.
Pharmacological Reports,
2016
.

Plyutinskaya A.D., Nemtsova E.R., Pankratov A.A., Shegai P.V., Krylov S.S., Iskandarova V.N., Maksimenko A.S., Demchuk D.V., Kuptsova T.S., Semenova M.N., Semenov V.V.
Bulletin of Experimental Biology and Medicine,
2022
.

Zaninetti R., Cortese S.V., Aprile S., Massarotti A., Canonico P.L., Sorba G., Grosa G., Genazzani A.A., Pirali T.
ChemMedChem,
2013
.

Burja B., Čimbora-Zovko T., Tomić S., Jelušić T., Kočevar M., Polanc S., Osmak M.
Bioorganic and Medicinal Chemistry,
2010
.

Biersack B., Effenberger K., Knauer S., Ocker M., Schobert R.
European Journal of Medicinal Chemistry,
2010
.

Stroylov V.S., Svitanko I.V., Maksimenko A.S., Kislyi V.P., Semenova M.N., Semenov V.V.
Bioorganic and Medicinal Chemistry Letters,
2020
.

Pati H.N., Wicks M., Holt Jr. H.L., LeBlanc R., Weisbruch P., Forrest L., Lee M.
Heterocyclic Communications,
2005
.

Shin K.D., Yoon Y.J., Kang Y., Son K., Kim H.M., Kwon B., Han D.C.
Biochemical Pharmacology,
2008
.

Sun C., Lin L., Yu H., Cheng C., Tsai Y., Chu C., Din Y., Chau Y., Don M.
Bioorganic and Medicinal Chemistry Letters,
2007