Keywords
antioxidant.
apiol
Baeyer–Villiger rearrangement
isoxazole
maleic anhydride
quinone
Abstract
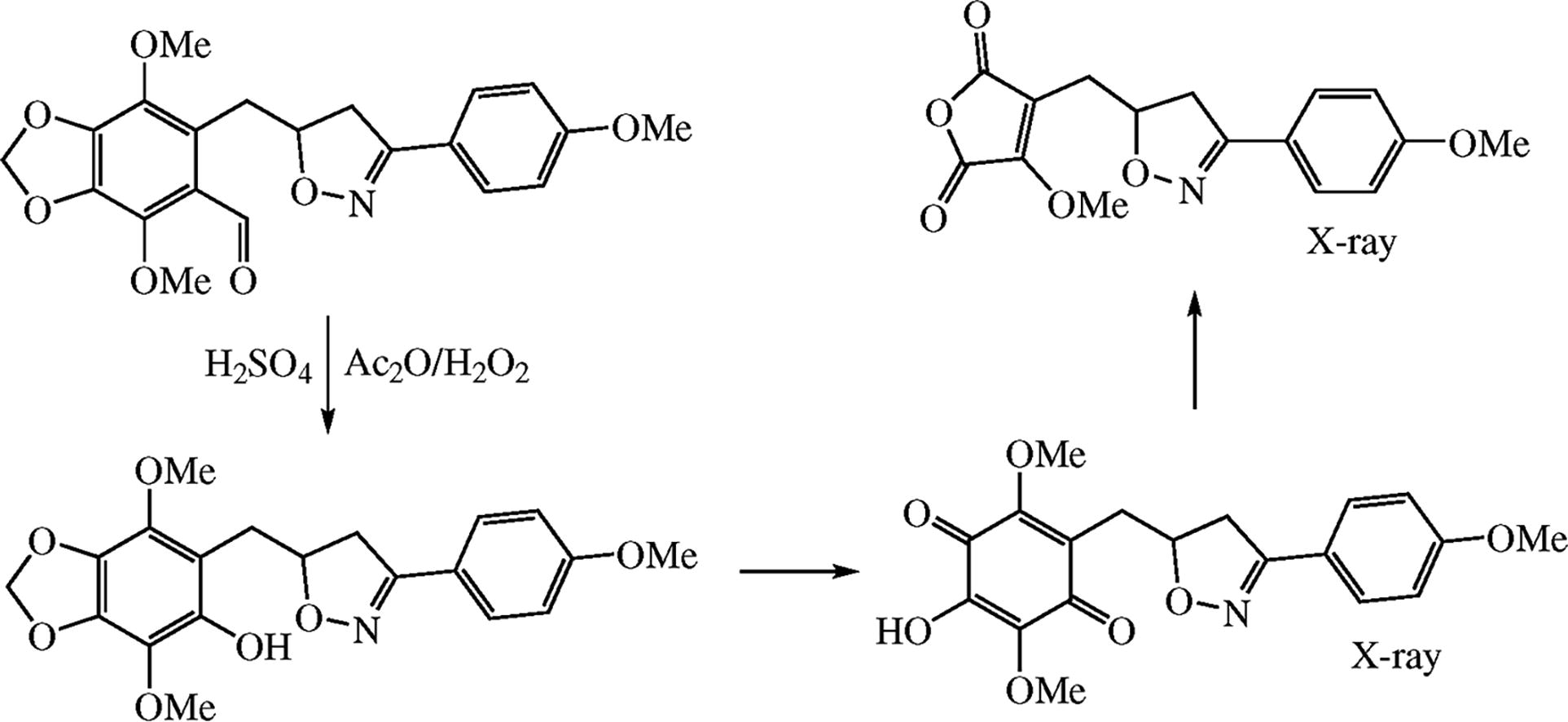
The Baeyer-Villiger oxidation of apiolaldehyde bearing o-(3-p-anisylisoxazolin-5-yl)methyl substituent proceeds first with the formation of the anticipated phenol. The subsequent oxidation of phenol with destruction of methylenedioxy ring leads to p-quinone derivative which would undergo opening of the benzene ring to finally produce maleic anhydride moiety. The structure of new compounds was proved by X-ray diffraction analysis.
References
.

Semenov V.V., Kiselyov A.S., Titov I.Y., Sagamanova I.K., Ikizalp N.N., Chernysheva N.B., Tsyganov D.V., Konyushkin L.D., Firgang S.I., Semenov R.V., Karmanova I.B., Raihstat M.M., Semenova M.N.
Journal of Natural Products,
2010
.

Semenov V.V., Rusak V.V., Chartov E.M., Zaretskii M.I., Konyushkin L.D., Firgang S.I., Chizhov A.O., Elkin V.V., Latin N.N., Bonashek V.M., Stas’eva O.N.
Russian Chemical Bulletin,
2007
.

Dallacker F.
Monatshefte fur Chemie,
1969
.

Capon R.J., Ghisalberti E.L., Jefferies P.R.
Phytochemistry,
1981
.
Tsyganov D.V., Demchuk D.V., Adaeva O.I., Konyushkin L.D., Minyaev M.E., Khrustalev V.N., Semenov V.V.
Mendeleev Communications,
2023
.

Ochi M., Kotsuki H., Inoue S., Taniguchi M., Tokoroyama T.
Chemistry Letters,
1979
.

Chai C.L., Elix J.A., Moore F.K.
Journal of Organic Chemistry,
2005
.

Tsyganov D.V., Krayushkin M.M., Konyushkin L.D., Strelenko Y.A., Semenova M.N., Semenov V.V.
Journal of Natural Products,
2016
.

Yoshioka K., Kamo S., Hosaka K., Sato R., Miikeda Y., Manabe Y., Tomoshige S., Tsubaki K., Kuramochi K.
ACS Omega,
2019
.

Hou Y., Vasileva E.A., Carne A., McConnell M., El-Din A. Bekhit A., Mishchenko N.P.
RSC Advances,
2018
.

Masuda K., Funayama S., Komiyama K., Unezawa I., Ito K.
Journal of Natural Products,
1987
.

Gubbens J., Zhu H., Girard G., Song L., Florea B., Aston P., Ichinose K., Filippov D., Choi Y., Overkleeft H., Challis G., van Wezel G.
Chemistry & Biology,
2014