Keywords
chiral vicinal diamines
dihydrobenzofurans
enantioselectivity
indolines
Palladium complexes
reductive Heck reaction
Abstract
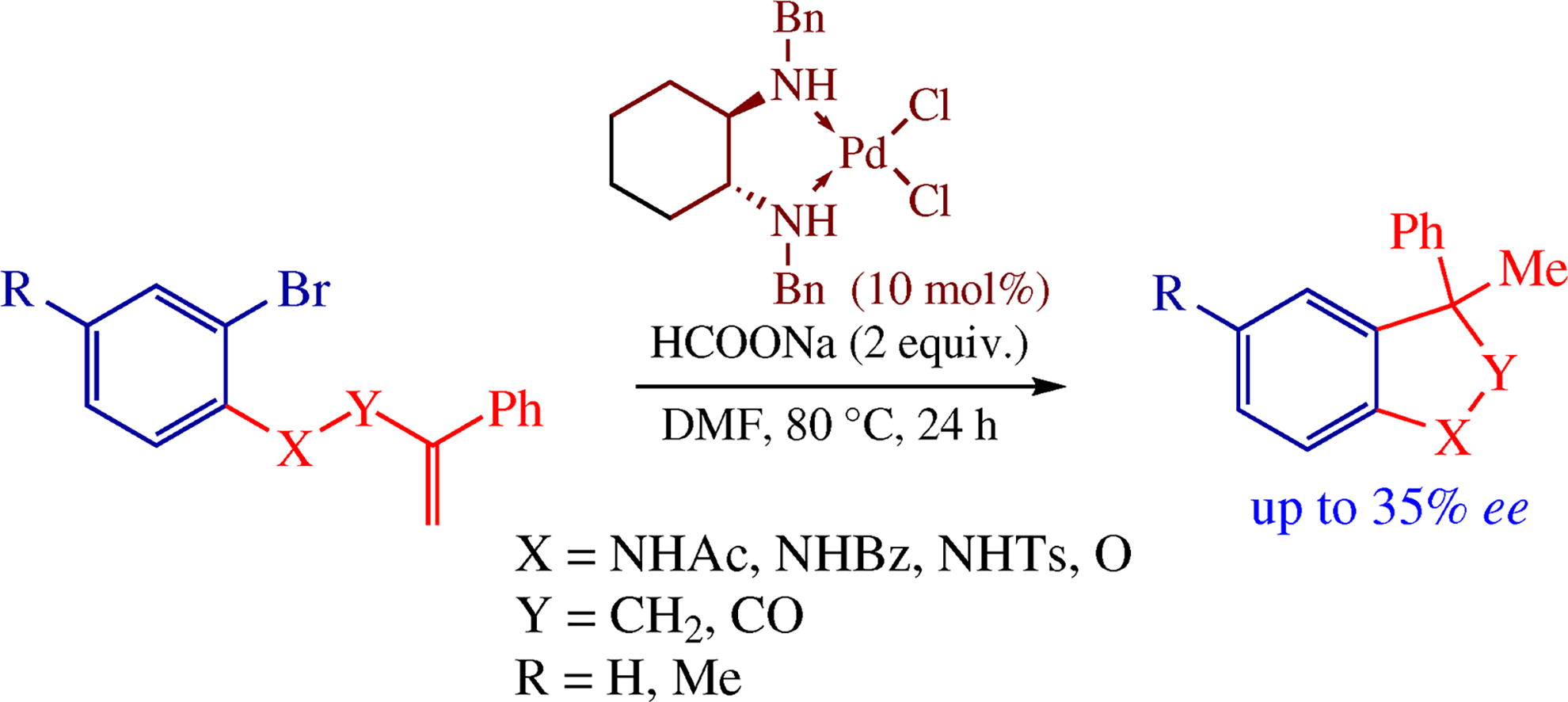
Palladium complexes with inexpensive and available vicinal diamines can serve as catalysts for the reductive Heck-type cyclization. o-Bromo-N-(2-phenylallyl)anilides are converted into the corresponding 3-methyl-3-phenylindolines, the analogous cyclization occurred for 2-bromo-4-methylphenyl 2-phenylallyl ether and N-(2-bromophenyl)-2-phenylacrylamides. The use of (1R,2R)-N,N’-dibenzylcyclohexane-1,2-diamine provides up to 35% ee of the indolines; this is the first case of asymmetric induction during the reductive Heck reaction involving diamine complexes.
References
1.

Larock R.C., Babu S.
Tetrahedron Letters,
1987
2.

Mc Cartney D., Guiry P.J.
Chemical Society Reviews,
2011
3.

Ghosh T.
ChemistrySelect,
2019
4.

Xie J., Liang R., Jia Y.
Chinese Journal of Chemistry,
2021
5.

Reznikov A.N., Ashatkina M.A., Klimochkin Y.N.
Organic and Biomolecular Chemistry,
2021
6.

Oxtoby L.J., Gurak J.A., Wisniewski S.R., Eastgate M.D., Engle K.M.
Trends in Chemistry,
2019
7.

Markies B.A., Canty A.J., de Graaf W., Boersma J., Janssen M.D., Hogerheide M.P., Smeets W.J., Spek A.L., van Koten G.
Journal of Organometallic Chemistry,
1994
8.
![Cationic Arylpalladium Complexes with Chelating Diamine Ligands, [PdAr(N−N)(solv)]BF4 (N−N = N,N,N‘,N‘-tetramethylethylenediamine, 2,2‘-bipyridine, 4,4‘-dimethyl-2,2‘-bipyridine). Preparation, Intermolecular Coupling of the Aryl Ligands, and Insertion of Alkyne and Allene into the Pd−C Bond](/storage/images/resized/iLiQsFqFaSEx6chlGQ5fbAwF6VYU3WWa08hkss0g_small_thumb.webp)
Yagyu T., Hamada M., Osakada K., Yamamoto T.
Organometallics,
2001
9.

Wilson J.E., Kurukulasuriya R., Reibarkh M., Reiter M., Zwicker A., Zhao K., Zhang F., Anand R., Colandrea V.J., Cumiskey A., Crespo A., Duffy R.A., Murphy B.A., Mitra K., Johns D.G., et. al.
ACS Medicinal Chemistry Letters,
2016
10.

Zeeli S., Weill T., Finkin-Groner E., Bejar C., Melamed M., Furman S., Zhenin M., Nudelman A., Weinstock M.
Journal of Medicinal Chemistry,
2018
11.

Yang X., Li D., Song A., Liu F.
Journal of Organic Chemistry,
2020
12.

Ma L., Tang L., Yi Q.
Frontiers in Pharmacology,
2019
13.

Sato R., Kanbara T., Kuwabara J.
Dalton Transactions,
2020
14.

Burns B., Grigg R., Sridharan V., Worakun T.
Tetrahedron Letters,
1988
15.

Kluwer A.M., Elsevier C.J., Bühl M., Lutz M., Spek A.L.
Angewandte Chemie - International Edition,
2003
16.

Diaz P., Phatak S., Xu J., Fronczek F., Astruc-Diaz F., Thompson C., Cavasotto C., Naguib M.
ChemMedChem,
2009
17.

Fu D., Li M., Zhang S., Li J., Sha B., Wang L., Zhang Y., Chen P., Hu T.
Bioorganic Chemistry,
2019
18.

Rakhit A., Hurley M.E., Tipnis V., Coleman J., Rommel A., Brunner H.R.
Journal of Clinical Pharmacology,
1986
19.

Wei H., Li B., Wang N., Ma Y., Yu J., Wang X., Su J., Liu D.
ChemistryOpen,
2023
20.

Diaz P., Gendre F., Stella L., Charpentier B.
Tetrahedron,
1998
21.

Yang X., Ma S., Du Y., Tao Y.
Chinese Journal of Organic Chemistry,
2013
22.
Kostyukovich A.Y., Gordeev E.G., Ananikov V.P.
Mendeleev Communications,
2023
23.

Abbas A.A., Dawood K.M.
RSC Advances,
2023
24.

Reznikov A.N., Ashatkina M.A., Yu. Vostruhina S., Klimochkin Y.N.
Tetrahedron Letters,
2023
25.
Kvashnin Y.A., Belyaev D.V., Kodess M.I., Ezhikova M.A., Rusinov G.L., Verbitskiy E.V., Charushin V.N.
Mendeleev Communications,
2023