Keywords
acetylenes
azines
ethynylation.
pyrazoles
superbases
Abstract
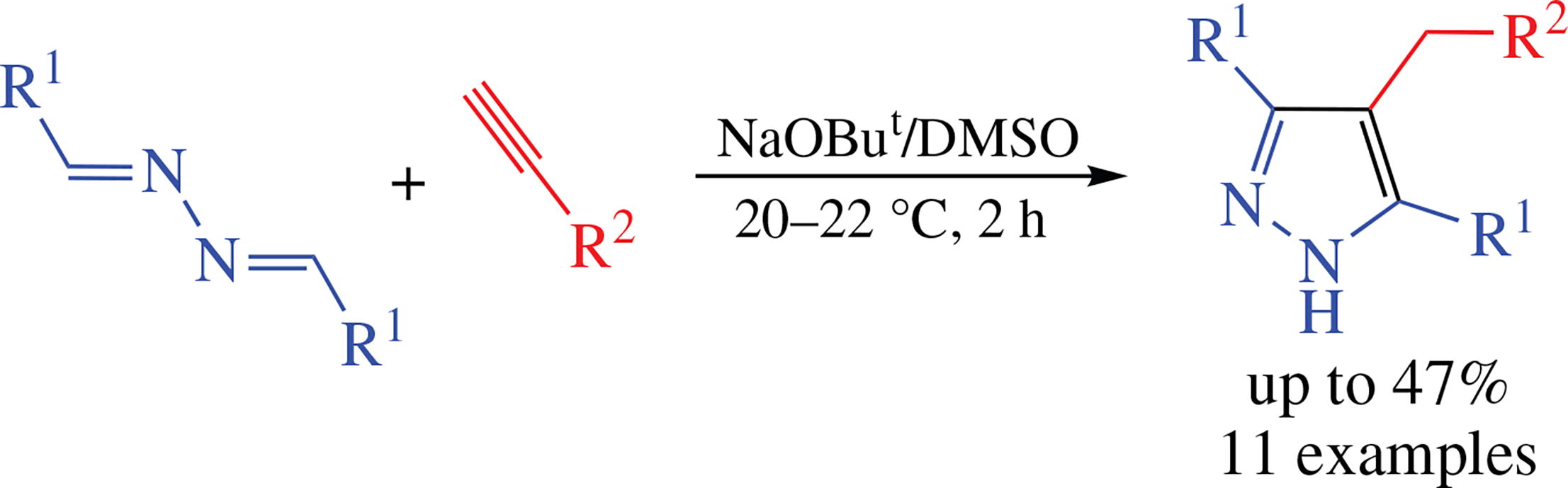
Arylacetylenes readily react with aromatic aldazines in superbase medium NaOBut/DMSO (1 vol% EtOH) at room temperature to afford mainly 4-arylmethyl-3,5-diaryl-1H- pyrazoles in up to 47% yield along with minor amounts of 1-arylmethyl-3,5-diaryl-1H-pyrazoles and 1,2-diaryl-1,2- bis(3,5-diaryl-1H-pyrazol-1-yl)ethanes. The reaction is rationalized as proceeding via the diazaallyl anions, the adducts of acetylenic carbanions to a C=N bond, which further undergo the proton transfer processes and intramolecular cyclization to the above pyrazole derivatives.
References
.

Transition-Metal-Free Superbase-Catalyzed C–H Vinylation of Aldimines with Acetylenes to 1-Azadienes
Schmidt E.Y., Bidusenko I.A., Protsuk N.I., Demyanov Y.V., Ushakov I.A., Vashchenko A.V., Trofimov B.A.
Journal of Organic Chemistry,
2020
.

Bidusenko I.A., Schmidt E.Y., Ushakov I.A., Trofimov B.A.
European Journal of Organic Chemistry,
2018
.

Trofimov B.A., Schmidt E.Y.
Accounts of Chemical Research,
2018
.

Bidusenko I.A., Schmidt E.Y., Ushakov I.A., Vashchenko A.V., Protsuk N.I., Orel V.B., Vitkovskaya N.M., Trofimov B.A.
Journal of Organic Chemistry,
2022
.

Voronin V.V., Ledovskaya M.S., Bogachenkov A.S., Rodygin K.S., Ananikov V.P.
Molecules,
2018
.

Gilmore K., Mohamed R.K., Alabugin I.V.
Wiley Interdisciplinary Reviews: Computational Molecular Science,
2016
.

Murakami K., Yamada S., Kaneda T., Itami K.
Chemical Reviews,
2017
.

Alabugin I.V., Gonzalez-Rodriguez E.
Accounts of Chemical Research,
2018
.

Gilmore K., Manoharan M., Wu J.I., Schleyer P.V., Alabugin I.V.
Journal of the American Chemical Society,
2012
.

Heravi M.M., Dehghani M., Zadsirjan V., Ghanbarian M.
Current Organic Synthesis,
2019
.

Chourasiya S.S., Kathuria D., Wani A.A., Bharatam P.V.
Organic and Biomolecular Chemistry,
2019
.

Syroeshkin M.A., Kuriakose F., Saverina E.A., Timofeeva V.A., Egorov M.P., Alabugin I.V.
Angewandte Chemie - International Edition,
2019
.

Dong K., Liu M., Xu X.
Molecules,
2022
.

Amariucai-Mantu D., Mangalagiu V., Bejan I., Aricu A., Mangalagiu I.I.
Pharmaceutics,
2022
.

Eckhardt P., Elliot Q., Alabugin I.V., Opatz T.
Chemistry - A European Journal,
2022
.

Potapov I.D., Voznarskiy A.Y., Mironov A.V., Motyakin M.V., Nekipelova T.D., Podrugina T.A.
Russian Chemical Bulletin,
2022
.

Ahmed J., Swain A.K., Das A., Govindarajan R., Bhunia M., Mandal S.K.
Chemical Communications,
2019
.

Zheng Q., Hua R., Jiang J., Zhang L.
Tetrahedron,
2014
.

Shah S., Das B.G., Singh V.K.
Tetrahedron,
2021
.

Hu C., Mena J., Alabugin I.V.
Nature Reviews Chemistry,
2023
.
Bidusenko I.A., Yu. Schmidt E., Protsuk N.I., Ushakov I.A., Trofimov B.A.
Mendeleev Communications,
2023
.

Josephitis C.M., Nguyen H.M., McNally A.
Chemical Reviews,
2023
.

Schmidt E.Y., Trofimov B.A.
Doklady Chemistry,
2022