Keywords
[4 + 2]-cycloaddition
1-(2-hydroxyaryl)ethanones
2,4,4-trimethylchromanes
crystal structure
dehydration
Grignard synthesis
Abstract
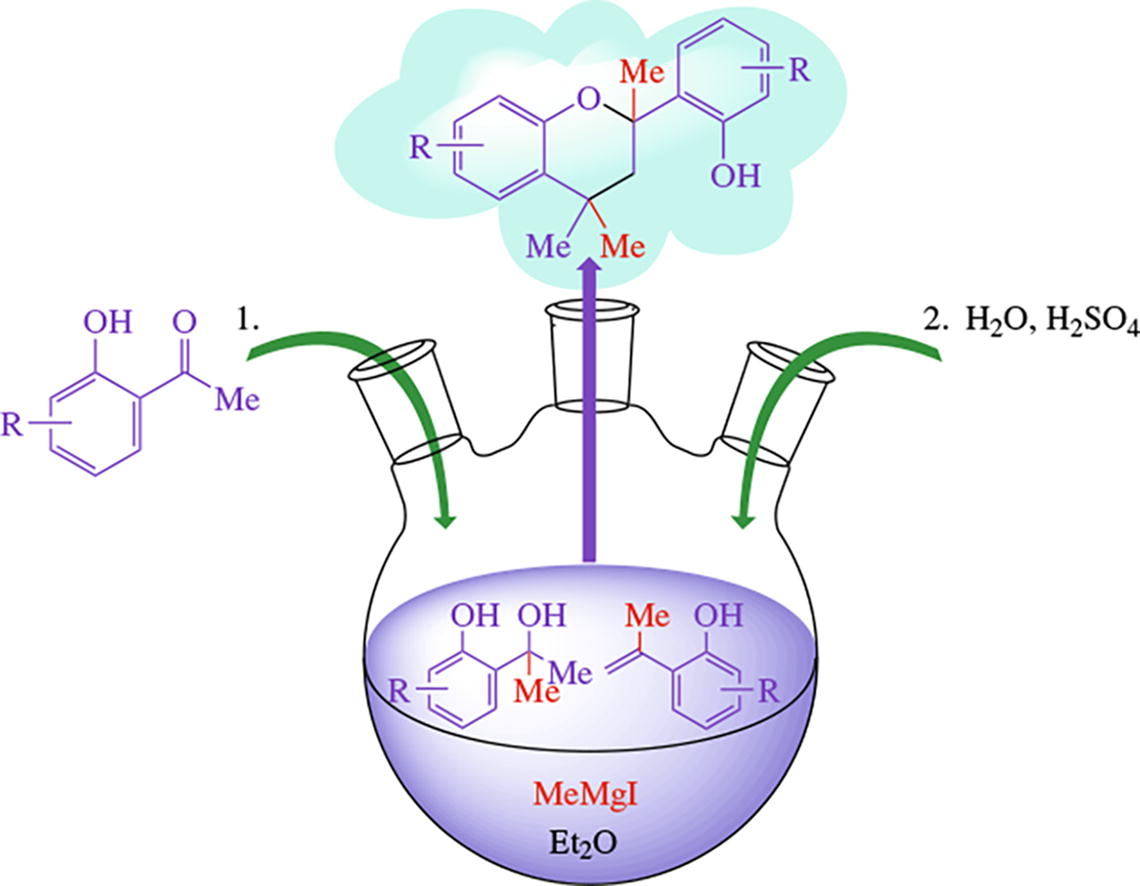
A one-pot synthesis of 2-(2-hydroxyaryl)-2,4,4-trimethyl-chromanes in near quantitative yields is based on the reaction of 1-(2-hydroxyaryl)ethanones with methylmagnesium iodide followed by treatment with dilute sulfuric acid. The structure of 2-(2-hydroxyphenyl)-2,4,4-trimethylchromane is confirmed by X-ray diffraction.
References
1.

Macrae C.F., Sovago I., Cottrell S.J., Galek P.T., McCabe P., Pidcock E., Platings M., Shields G.P., Stevens J.S., Towler M., Wood P.A.
Journal of Applied Crystallography,
2020
2.

Farrugia L.J.
Journal of Applied Crystallography,
2012
3.

Ullah A., Munir S., Badshah S.L., Khan N., Ghani L., Poulson B.G., Emwas A., Jaremko M.
Molecules,
2020
4.

Zhen X., Quan Y., Peng Z., Han Y., Zheng Z., Guan L.
Chemical Biology and Drug Design,
2016
5.

BAKER W., BESLY D.M.
Nature,
1939
6.

Cheng N., Yi W., Wang Q., Peng S., Zou X.
Chinese Chemical Letters,
2014
7.

Dongamanti A., Aamate V.K., Devulapally M.G., Gundu S., Kotni M.K., Manga V., Balasubramanian S., Ernala P.
Bioorganic and Medicinal Chemistry Letters,
2015
8.
Tsyganov D.V., Konyushkin L.D., Semenova M.N., Semenov V.V.
Mendeleev Communications,
2016
9.

Wei Z., Chi K., Yu Z., Liu H., Sun L., Zheng C., Piao H.
Bioorganic and Medicinal Chemistry Letters,
2016
10.

Hatnapure G.D., Keche A.P., Rodge A.H., Birajdar S.S., Tale R.H., Kamble V.M.
Bioorganic and Medicinal Chemistry Letters,
2012
11.

Lewin G., Cojean S., Gupta S., Verma A., Puri S.K., Loiseau P.M.
Biomedicine & Preventive Nutrition,
2011
12.

Casano G., Dumètre A., Pannecouque C., Hutter S., Azas N., Robin M.
Bioorganic and Medicinal Chemistry,
2010
13.

Aoyama T., Yamamoto T., Miyota S., Hayakawa M., Takido T., Kodomari M.
Synlett,
2014
14.

Naithani R., Huma L., Holland L., Shukla D., McCormick D., Mehta R., Moriarty R.
Mini-Reviews in Medicinal Chemistry,
2008
15.
Filimonov S.I., Savinsky N.G., Evstigneeva E.M.
Mendeleev Communications,
2003
16.
Prusek O., Bureš F., Pytela O.
Collection of Czechoslovak Chemical Communications,
2009
17.

Osyanin V.A., Lukashenko A.V., Osipov D.V.
Russian Chemical Reviews,
2021
18.

19.

Çetinkaya S., Taban Akça K., Süntar I.
Studies in Natural Products Chemistry,
2022