Keywords
1-aroylmethylidene-1
2
2-(pyrazol-3-yl)-O-methylserotonins
2-(pyrazol-3-yl)tryptamine
3
4-tetrahydro-β-carbolines
pyrazolylmelatonins
quantum chemical calculations.
recyclization
tryptamines
β-carbolines
Abstract
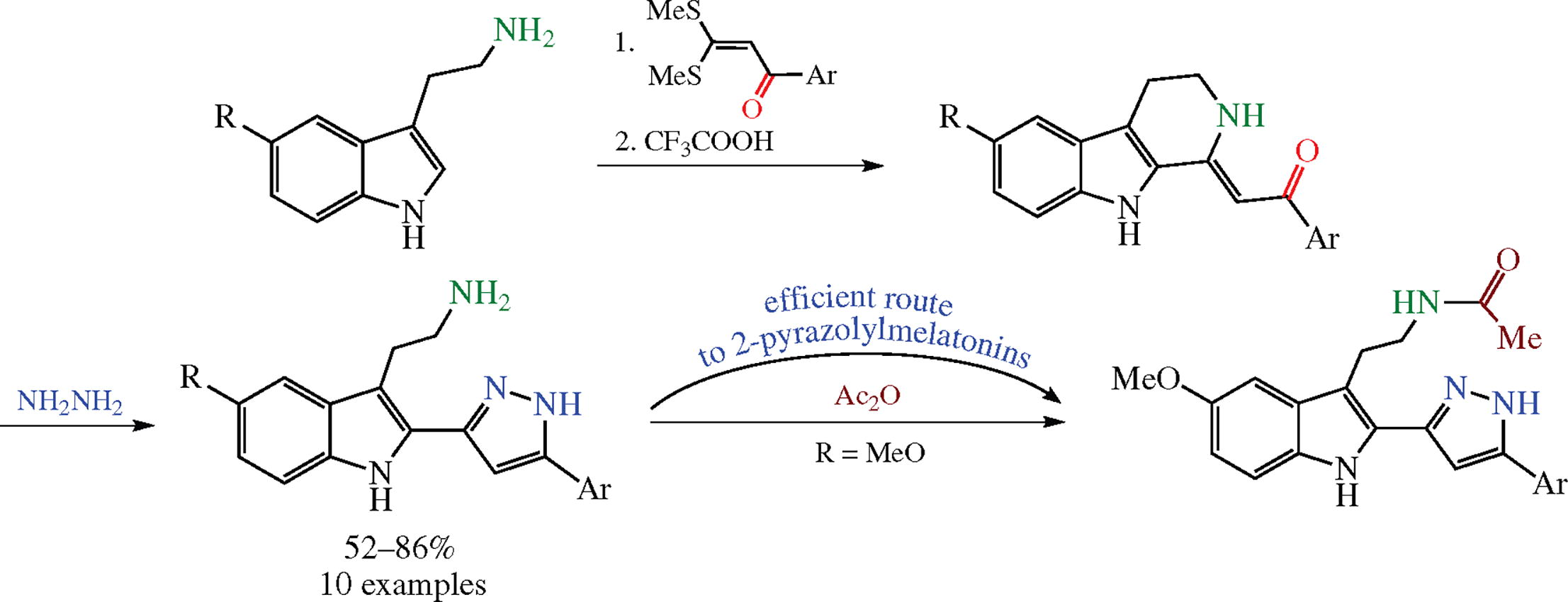
A cyclization-recyclization pathway for indirect 2-heteroarylation of tryptamines has been suggested by the example of introducing the pyrazolyl moiety. The process involves the intermediate cyclization of tryptamines into push-pull type β-carboline semi-products. The relative stability of the tautomeric forms of 2-pyrazolyltryptamines has been estimated using the DFT method.
References
.

.

Dar'in D.V., Lobanov P.S.
Russian Chemical Reviews,
2015
.
![Aza-Annulation of 1,2,3,4-Tetrahydro-β-carboline Derived Enaminones and Nitroenamines: Synthesis of Functionalized Indolizino[8,7-b]indoles, Pyrido[1,2-a:3,4-b′]diindoles, Indolo[2,3-a]quinolizidine-4-ones and Other Tetrahydro-β-carboline Fused Heterocycles](/storage/images/resized/iLiQsFqFaSEx6chlGQ5fbAwF6VYU3WWa08hkss0g_small_thumb.webp)
Avadhani A., Iniyavan P., Acharya A., Gautam V., Chakrabarti S., Ila H.
ACS Omega,
2019
.

Zubenko A.A., Kartsev V.G., Morkovnik A.S., Divaeva L.N., Suponitsky K.Y.
ChemistrySelect,
2016
.

Fabitha K., Chandrakanth M., Pramod R.N., Arya C.G., Li Y., Banothu J.
ChemistrySelect,
2022
.

The case of tryptamine and serotonin in plants: a mysterious precursor for an illustrious metabolite
Negri S., Commisso M., Avesani L., Guzzo F.
Journal of Experimental Botany,
2021
.

Zubenko A.A., Morkovnik A.S., Divaeva L.N., Kartsev V.G., Anisimov A.A., Suponitsky K.Y.
Russian Journal of Organic Chemistry,
2019
.

Zhang L., Peslherbe G.H., Muchall H.M.
Canadian Journal of Chemistry,
2010
.
Zubenko A.A., Morkovnik A.S., Divaeva L.N., Kartsev V.G., Borodkin G.S., Klimenko A.I.
Mendeleev Communications,
2018
.

Hegazy A., Mahmoud S.H., Elshaier Y.A., Shama N.M., Nasr N.F., Ali M.A., El-Shazly A.M., Mostafa I., Mostafa A.
Scientific Reports,
2023
.
Zubenko A.A., Sochnev V.S., Kartsev V.G., Divaeva L.N., Demidov O.P., Klimenko A.I., Bodryakov A.N., Bodryakova M.A., Borodkin G.S., Morkovnik A.S.
Mendeleev Communications,
2021
.

Luo B., Song X.
European Journal of Medicinal Chemistry,
2021
.

Giroud C., van der Leer T., van der Heijden R., Verpoorte R., Heeremans C., Niessen W., vander Greef J.
Planta Medica,
1991
.
Zubenko A.A., Morkovnik A.S., Divaeva L.N., Sochnev V.S., Demidov O.P., Fetisov L.N., Andros N.O., Svyatogorova A.E., Klimenko A.I.
Mendeleev Communications,
2023
.

Puerto Galvis C.E., Kouznetsov V.V.
Studies in Natural Products Chemistry,
2018