Keywords
Curtius rearrangement
Knoevenagel reaction
plant allyltetraalkoxybenzenes
polyalkoxyquinoline derivatives
tetraalkoxybenzaldehydes
Vilsmeier reaction.
Abstract
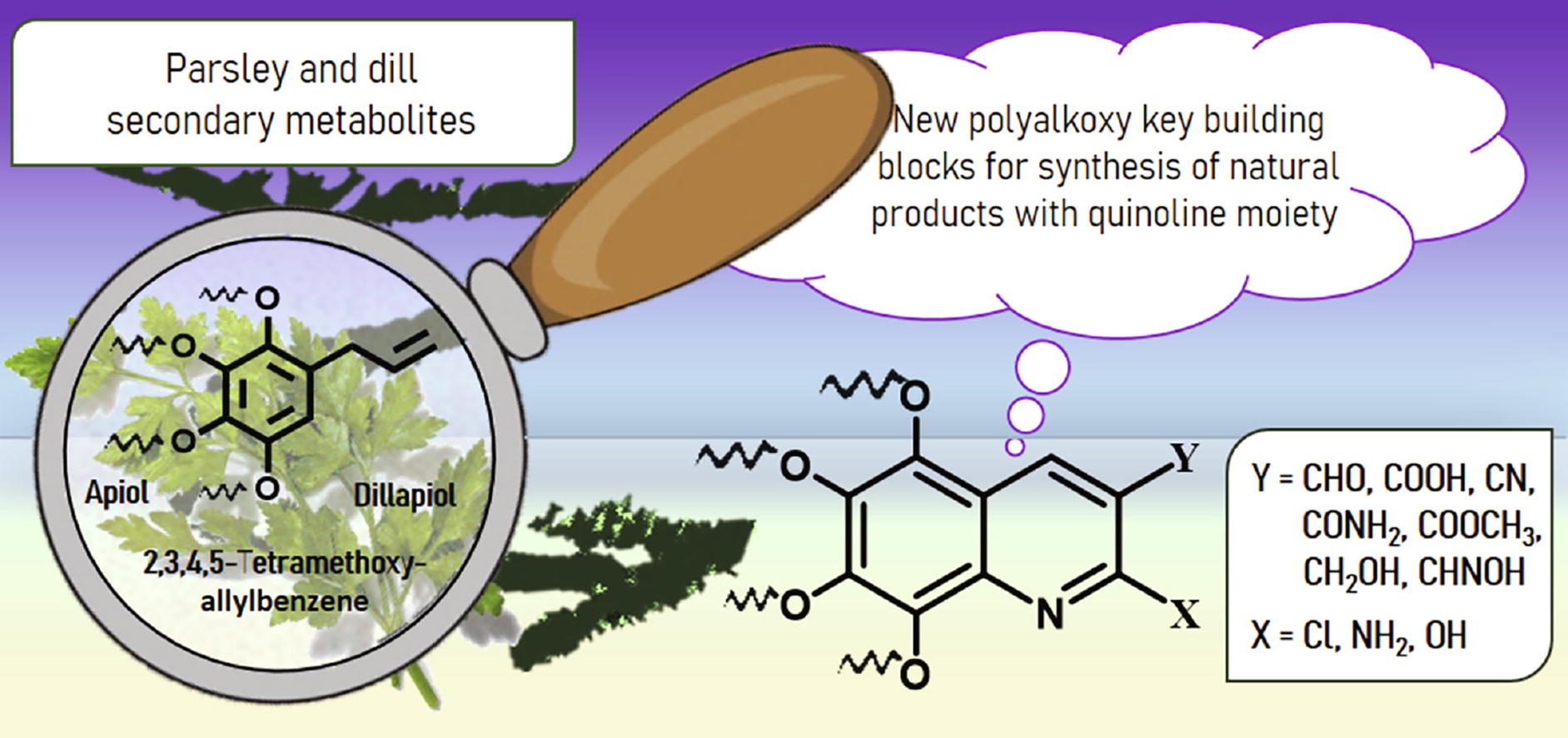
Efficient preparative procedures for the synthesis of 2,3-disubstituted 5,6,7,8-tetraalkoxyquinolines starting from tetraalkoxybenzaldehydes are reported. The latter are available from plant (polyalkoxy)(allyl)benzenes, secondary metabolites of parsley and dill seeds.
References
.

Gill R., Kaur R., Kaur G., Rawal R., Shah A., Bariwal J.
Current Organic Chemistry,
2014
.

Semenov V.V., Kiselyov A.S., Titov I.Y., Sagamanova I.K., Ikizalp N.N., Chernysheva N.B., Tsyganov D.V., Konyushkin L.D., Firgang S.I., Semenov R.V., Karmanova I.B., Raihstat M.M., Semenova M.N.
Journal of Natural Products,
2010
.

Shestopalov A.M., Litvinov Y.M., Rodinovskaya L.A., Malyshev O.R., Semenova M.N., Semenov V.V.
ACS Combinatorial Science,
2012
.

Semenova M.N., Tsyganov D.V., Malyshev O.R., Ershov O.V., Bardasov I.N., Semenov R.V., Kiselyov A.S., Semenov V.V.
Bioorganic and Medicinal Chemistry Letters,
2014
.

Karad S.C., Purohit V.B., Thummar R.P., Vaghasiya B.K., Kamani R.D., Thakor P., Thakkar V.R., Thakkar S.S., Ray A., Raval D.K.
European Journal of Medicinal Chemistry,
2017
.

Bindu P.J., Mahadevan K.M., Satyanarayan N.D., Ravikumar Naik T.R.
Bioorganic and Medicinal Chemistry Letters,
2012
.

Prakash Chaturvedula V.S., Schilling J.K., Miller J.S., Andriantsiferana R., Rasamison V.E., Kingston D.G.
Journal of Natural Products,
2003
.

.

Kopustinskiene D.M., Jakstas V., Savickas A., Bernatoniene J.
Nutrients,
2020
.

Karatoprak G.Ş., Küpeli Akkol E., Genç Y., Bardakcı H., Yücel Ç., Sobarzo-Sánchez E.
Molecules,
2020
.

Elban M.A., Sun W., Eisenhauer B.M., Gao R., Hecht S.M.
Organic Letters,
2006
.

Potapov A.Y., Paponov B.V., Podoplelova N.A., Panteleev M.A., Polikarchuk V.A., Ledenyova I.V., Stolpovskaya N.V., Kryl’skii D.V., Shikhaliev K.S.
Russian Chemical Bulletin,
2021
.
![Efficient one-pot synthesis of new 1-amino substituted pyrrolo[1,2-a]quinoline-4-carboxylate esters via copper-free Sonogashira coupling reactions](/storage/images/resized/voXLqlsvTwv5p3iMQ8Dhs95nqB4AXOG7Taj7G4ra_small_thumb.webp)
Keivanloo A., Kazemi S.S., Nasr-Isfahani H., Bamoniri A.
Molecular Diversity,
2016
.

Kumar S., Bawa S., Gupta H.
Mini-Reviews in Medicinal Chemistry,
2009
.

BALITZ D.M., BUSH J.A., BRADNER W.T., DOYLE T.W., O''HERRON F.A., NETTLETON D.E.
Journal of Antibiotics,
2012
.

Bolzán A.D., Bianchi M.S.
Mutation Research - Reviews in Mutation Research,
2001
.

Mahmoudian M., Rahimi-Moghaddam P.
Recent Patents on Anti-Cancer Drug Discovery,
2008
.
Karmanova I.B., Firgang S.I., Konyushkin L.D., Khrustalev V.N., Ignatov A.V., Kuznetsov L.A., Pinchuk Y.A., Kozlov I.A., Semenov V.V.
Mendeleev Communications,
2016
.
Tsyganov D.V., Konyushkin L.D., Semenova M.N., Semenov V.V.
Mendeleev Communications,
2016
.

Chernysheva N.B., Tsyganov D.V., Philchenkov A.A., Zavelevich M.P., Kiselyov A.S., Semenov R.V., Semenova M.N., Semenov V.V.
Bioorganic and Medicinal Chemistry Letters,
2012
.
Silyanova E.A., Ushkarov V.I., Samet A.V., Maksimenko A.S., Koblov I.A., Kislyi V.P., Semenova M.N., Semenov V.V.
Mendeleev Communications,
2022
.

Hou Y., Harinantenaina L.
Current Medicinal Chemistry,
2010
.

Teodor E.D., Ungureanu O., Gatea F., Radu G.L.
Anti-Cancer Agents in Medicinal Chemistry,
2020
.

Semenov V.V., Semenova M.N.
Russian Chemical Reviews,
2015