Keywords
14-prostaglandin J2
15-deoxyΔ12
Corey lactone diol
dehydration
diastereoselective reaction
dienes.
prostaglandin J2 methyl ester
Abstract
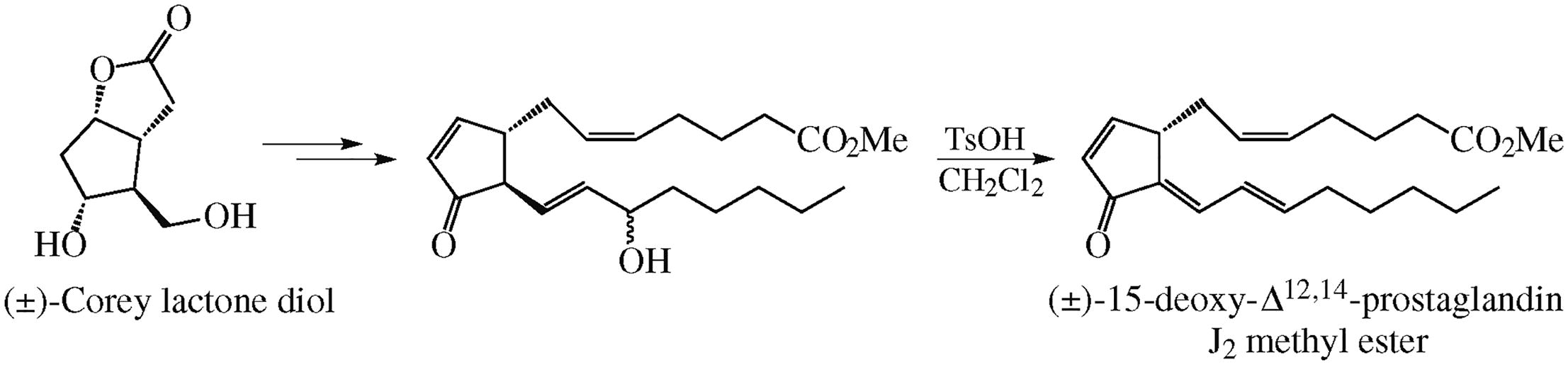
A simple and facile synthesis of racemic 15-deoxy-Δ12,14-prostaglandin J2 methyl ester from readily available Corey lactone diol in total eleven steps was suggested. The standard methods provided a pathway to a block with an integrated w w w-chain and further to PGJ2 methyl ester. The latter was smoothly converted to the target prostaglandin in the TsOH-CH2Cl2 medium when allylic alcohol moiety was transformed to exocyclic diene substituent conjugated with endocyclic enone system.
References
.

Kim N., Moon H., Park T., Yun H., Jung J., Chang D., Kim D., Suh Y.
Journal of Organic Chemistry,
2010
.

Suzuki M., Mori M., Niwa T., Hirata R., Furuta K., Ishikawa T., Noyori R.
Journal of the American Chemical Society,
1997
.

Brummond K.M., Sill P.C., Chen H.
Organic Letters,
2003
.

Zanoni G., Porta A., De Toma Q., Castronovo F., Vidari G.
Journal of Organic Chemistry,
2003
.

Brunoldi E.M., Zanoni G., Vidari G., Sasi S., Freeman M.L., Milne G.L., Morrow J.D.
Chemical Research in Toxicology,
2007
.

Uchida K., Shibata T.
Chemical Research in Toxicology,
2007
.

Egger J., Fischer S., Bretscher P., Freigang S., Kopf M., Carreira E.M.
Organic Letters,
2015
.

Li J., Ahmed T.S., Xu C., Stoltz B.M., Grubbs R.H.
Journal of the American Chemical Society,
2018
.

Kudva A.K., Kaushal N., Mohinta S., Kennett M.J., August A., Paulson R.F., Prabhu K.S.
PLoS ONE,
2013
.

Loza V.V., Gimazetdinov A.M., Miftakhov M.S.
Russian Journal of Organic Chemistry,
2018
.

Vostrikov N.S., Lobko I.F., Ishimova D.U., Miftakhov M.S.
Russian Journal of Organic Chemistry,
2015
.

Iqbal M., Duffy P., Evans P., Cloughley G., Allan B., Lledó A., Verdaguer X., Riera A.
Organic and Biomolecular Chemistry,
2008
.
![Development of a new approach for the synthesis of (+)-15-deoxy-Δ12,14-prostaglandin J2 methyl ester based on the [2+2]-cycloadduct of 5-trimethylsilylcyclopentadiene and dichloroketene](/storage/images/resized/leiAYcRDGTSl5B1eCnwpSGqmDEUEfDPPoYisFGhT_small_thumb.webp)
Gimazetdinov A.M., Al’mukhametov A.Z., Miftakhov M.S.
New Journal of Chemistry,
2022
.

Baxter A.D., Binns F., Javed T., Roberts S.M., Sadler P., Scheinmann F., Wakefield B.J., Lynch M., Newton R.F.
Journal of the Chemical Society Perkin Transactions 1,
1986
.

Straus D.S., Glass C.K.
Medicinal Research Reviews,
2001
.

Acharya H.P., Kobayashi Y.
Tetrahedron Letters,
2004
.

Acharya H.P., Kobayashi Y.
Tetrahedron,
2006
.

Kim E., Surh Y.
Biochemical Pharmacology,
2006