Keywords
(acylethynyl)pyrroles
deacylation
deprotection
ethynylpyrroles
potassium tert-butoxide.
pyrroles
terminal alkynes
ynones
Abstract
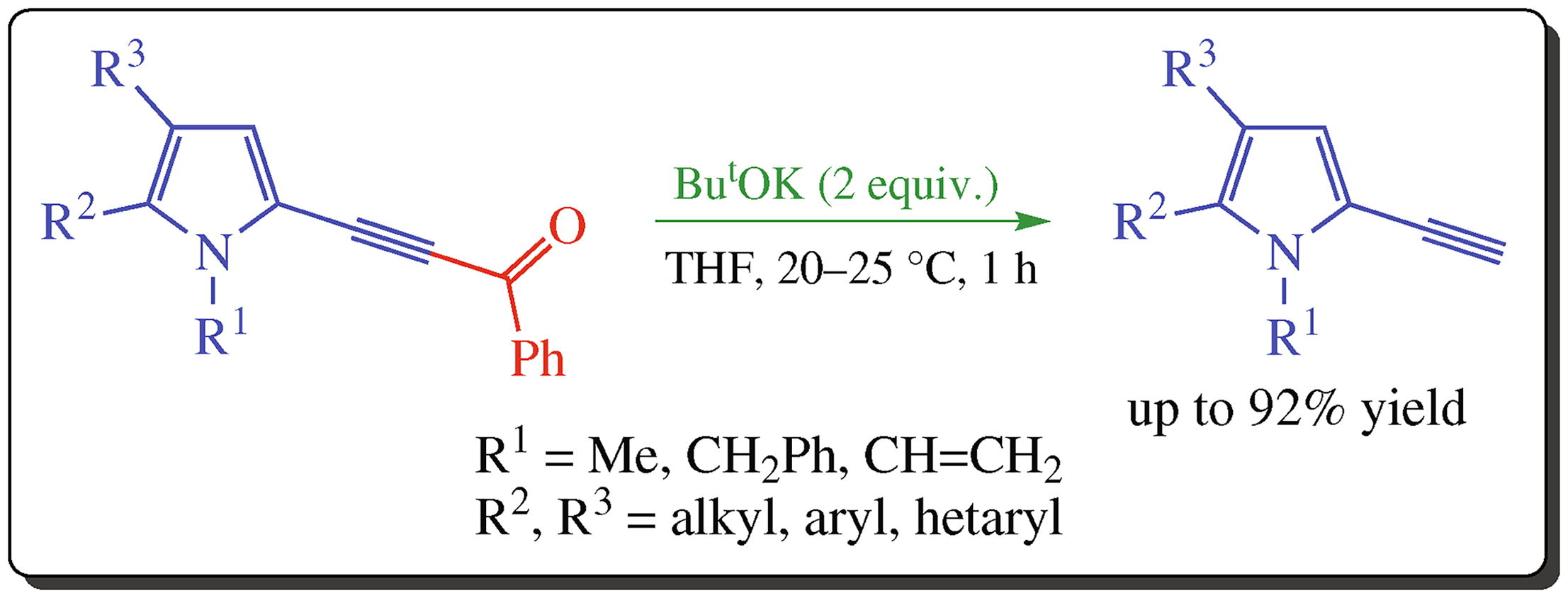
Available N-substituted 2-(acylethynyl)pyrroles undergo room temperature deprotection in the ButOK/THF system to give 2-ethynylpyrroles in 82-92% yields. Quantum-chemical calculations (B2PLYP/6-311G**//B3LYP/6-311G**+C-PCM/THF) show that the cleavage of ethynyl-acyl bond via the proton transfer from ButOK with formation of potassium acylate and 2-methylpropene is thermo-dynamically much more preferred compared to alternative nucleophilic attack of tert-butoxide anion at the acyl carbon (ΔG = -35.1 vs. -2.7 kcal mol-1).
References
.

Yasuda T., Imase T., Nakamura Y., Yamamoto T.
Macromolecules,
2005
.

Merkul E., Boersch C., Frank W., Müller T.J.
Organic Letters,
2009
.

Trofimov B.A., Stepanova Z.V., Sobenina L.N., Mikhaleva A.I., Ushakov I.A.
Tetrahedron Letters,
2004
.

Tu B., Ghosh B., Lightner D.A.
Monatshefte fur Chemie,
2004
.

Liao J., Chen C., Chou H., Cheng C., Chou P., Fang J., Slanina Z., Chow T.J.
Organic Letters,
2002
.

Sobenina L.N., Trofimov B.A.
Molecules,
2020
.

Bitar A.Y., Frontier A.J.
Organic Letters,
2008
.

Sessler J.L., Cai J., Gong H., Yang X., Arambula J.F., Hay B.P.
Journal of the American Chemical Society,
2010
.
![The Dual Role of 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) in the Synthesis of Terminal Aryl- and Styryl-Acetylenes via Umpolung Reactivity](/storage/images/resized/iLiQsFqFaSEx6chlGQ5fbAwF6VYU3WWa08hkss0g_small_thumb.webp)
Morri A.K., Thummala Y., Doddi V.R.
Organic Letters,
2015
.

Saito K., Yoshida M., Uekusa H., Doi T.
ACS Omega,
2017
.

Cheema H., Baumann A., Loya E.K., Brogdon P., McNamara L.E., Carpenter C.A., Hammer N.I., Mathew S., Risko C., Delcamp J.H.
ACS applied materials & interfaces,
2019
.

Rana A., Lee S., Kim D., Panda P.K.
Chemistry - A European Journal,
2015
.

Debnath S., Singh S., Bedi A., Krishnamoorthy K., Zade S.S.
Journal of Polymer Science, Part A: Polymer Chemistry,
2016
.

Liu C., Dai R., Yao G., Deng Y.
Journal of Chemical Research,
2014
.

Sobenina L.N., Tomilin D.N., Petrova O.V., Gulia N., Osowska K., Szafert S., Mikhaleva A.I., Trofimov B.A.
Russian Journal of Organic Chemistry,
2010
.

Smith J.A., Ng S., White J.
Organic and Biomolecular Chemistry,
2006
.

Heynderickx A., Mohamed Kaou A., Moustrou C., Samat A., Guglielmetti R.
New Journal of Chemistry,
2003
.

D'hooghe M., Buyck C., Contreras J., De Kimpe N.
Organic and Biomolecular Chemistry,
2008
.

Guérin C., Jean-Gérard L., Octobre G., Pascal S., Maury O., Pilet G., Ledoux A., Andrioletti B.
RSC Advances,
2015
.

Lim J.Y., Beer P.D.
New Journal of Chemistry,
2018
.

Lee C., Lee H., Lee S., Jeon H., Jeong K.
Organic Chemistry Frontiers,
2019
.

Choudhary S., Yadav J., Mamta, Pawar A.P., Vanaparthi S., Mir N.A., Iype E., Sharma R., Kant R., Kumar I.
Organic and Biomolecular Chemistry,
2020
.

Martire D.O., Jux N., Aramendia P.F., Martin Negri R., Lex J., Braslavsky S.E., Schaffner K., Vogel E.
Journal of the American Chemical Society,
1992
.

D’Auria M., De Luca E., Mauriello G., Racioppi R., Sleiter G.
Journal of the Chemical Society Perkin Transactions 1,
1997
.
Negishi E., Xu C., Tan Z., Kotora M.
Heterocycles,
1997
.

Tanaka Y., Ishisaka T., Koike T., Akita M.
Polyhedron,
2015
.

Tomilin D.N., Gotsko M.D., Sobenina L.N., Ushakov I.A., Afonin A.V., Soshnikov D.Y., Trofimov A.B., Koldobsky A.B., Trofimov B.A.
Journal of Fluorine Chemistry,
2016
.

Yoshida M., Easmin S., Al-Amin M., Hirai Y., Shishido K.
Tetrahedron,
2011
.

Kitano Y., Suzuki T., Kawahara E., Yamazaki T.
Bioorganic and Medicinal Chemistry Letters,
2007
.

Tietze L.F., Kettschau G., Heitmann K.
Synthesis,
1996
.

Haubmann C., Hübner H., Gmeiner P.
Bioorganic and Medicinal Chemistry Letters,
1999
.

Tomilin D.N., Sobenina L.N., Belogolova A.M., Trofimov A.B., Ushakov I.A., Trofimov B.A.
Molecules,
2023