Keywords
charge
cytotoxicity.
L-cysteine
nanoparticles
self-assembly
silver
size
Abstract
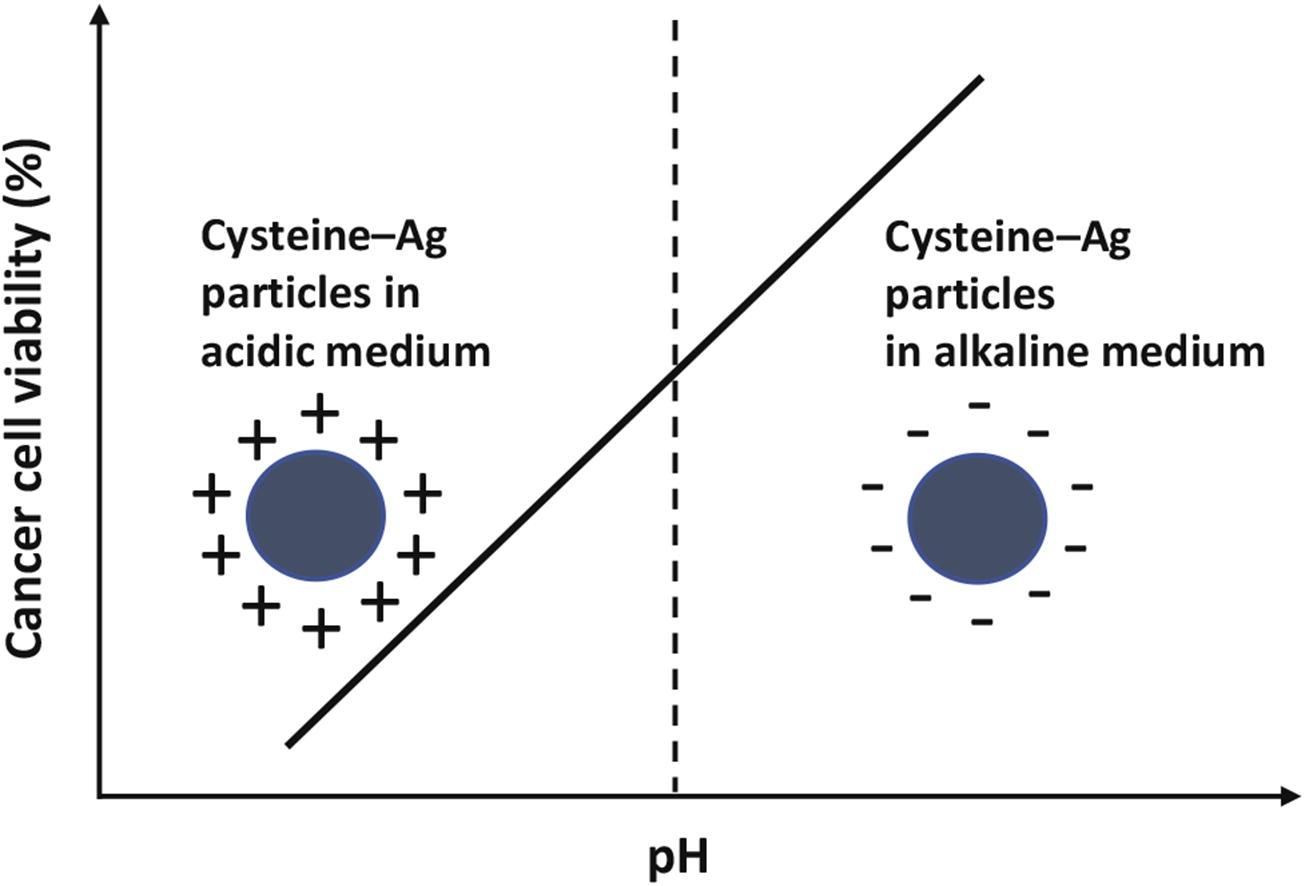
Polypeptide-like supramolecular aggregates are formed in aqueous cysteine-AgNO3 solution. At low pH, their positively charged shell consists of several layers of cysteine-Ag+ complexes held by hydrogen bonds; with an increase in pH, the particles aggregate at isoelectric point; while at higher pH, the shell acquires negative charge and starts being destroyed. The pH control has been demonstrated for cytotoxic effect of the solution against MCF-7 breast cancer cells
References
.

Samai S., Dey J., Biradha K.
Soft Matter,
2011
.

Zou A., Li Y., Chen Y., Angelova A., Garamus V.M., Li N., Drechsler M., Angelov B., Gong Y.
Colloids and Surfaces B: Biointerfaces,
2017
.

Suzuki M., Hanabusa K.
Chemical Society Reviews,
2009
.

Shen J., Li D., Zhang M., Zhou J., Zhang H., Jiang Y.
Langmuir,
2010
.

Du X., Zhou J., Shi J., Xu B.
Chemical Reviews,
2015
.

Draper E.R., Adams D.J.
Langmuir,
2019
.

Hu B., Yan H., Sun Y., Chen X., Sun Y., Li S., Jing Y., Li H.
Artificial Cells, Nanomedicine and Biotechnology,
2019
.

Odriozola I., Loinaz I., Pomposo J.A., Grande H.J.
Journal of Materials Chemistry A,
2007
.

Casuso P., Carrasco P., Loinaz I., Grande H.J., Odriozola I.
Organic and Biomolecular Chemistry,
2010
.

Casuso P., Carrasco P., Loinaz I., Cabañero G., Grande H.J., Odriozola I.
Soft Matter,
2011
.

Li P., Dou X., Feng C., Zhang D.
Soft Matter,
2013
.

Casuso P., Pérez-San Vicente A., Iribar H., Gutiérrez-Rivera A., Izeta A., Loinaz I., Cabañero G., Grande H., Odriozola I., Dupin D.
Chemical Communications,
2014
.

Kubiak P.S., Awhida S., Hotchen C., Deng W., Alston B., McDonald T.O., Adams D.J., Cameron P.J.
Chemical Communications,
2015
.

Khizhnyak S.D., Komarov P.V., Ovchinnikov M.M., Zherenkova L.V., Pakhomov P.M.
Soft Matter,
2017
.

Vishnevetskii D.V., Mekhtiev A.R., Perevozova T.V., Averkin D.V., Ivanova A.I., Khizhnyak S.D., Pakhomov P.M.
Soft Matter,
2020
.

Vishnevetskii D.V., Averkin D.V., Efimov A.A., Lizunova A.A., Ivanova A.I., Pakhomov P.M., Ruehl E.
Soft Matter,
2021
.

Vishnevetskii D.V., Mekhtiev A.R., Perevozova T.V., Ivanova A.I., Averkin D.V., Khizhnyak S.D., Pakhomov P.M.
Soft Matter,
2022
.

Restu W.K., Nishida Y., Kataoka T., Morimoto M., Ishida K., Mizuhata M., Maruyama T.
Colloid and Polymer Science,
2017
.

Mukherjee S., Kar T., Kumar Das P.
Chemistry - An Asian Journal,
2014
.

Xiong W., Zhou H., Zhang C., Lu H.
Chinese Chemical Letters,
2017
.

Fernando I., Zhou Y.
Chemosphere,
2019