Keywords
alcohols
deoxygenation
ethers.
organofluorine compounds
photocatalysis
radicals
Abstract
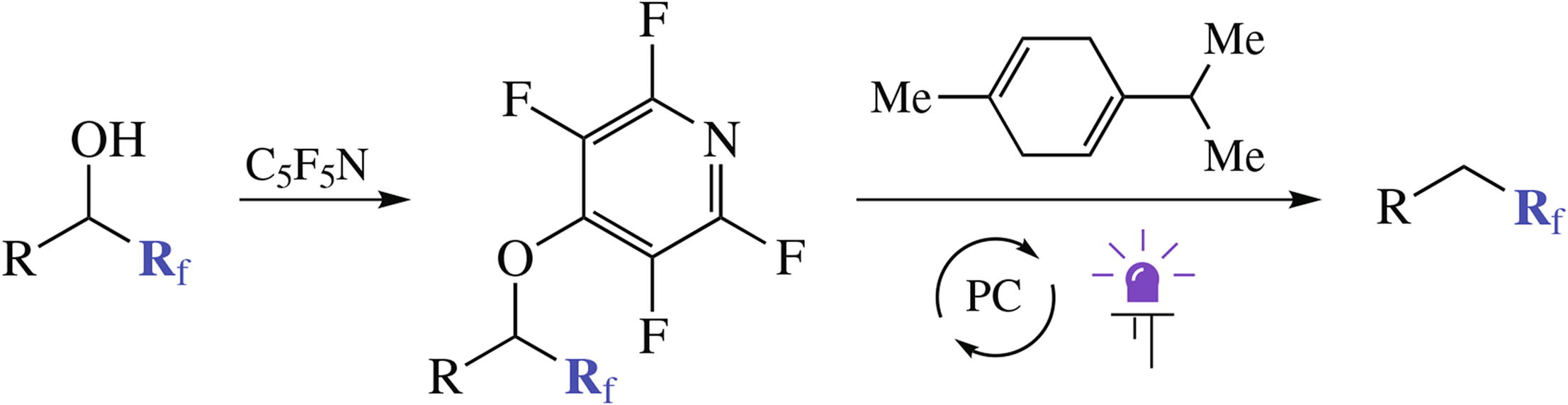
A new method for deoxygenation of fluoroalkyl-substituted alcohols involves derivatization of the hydroxy group with pentafluoropyridine followed by photoredox catalyzed reduction of the obtained hetaryl ethers using γ-terpinene as a source of hydrogen. The initial alcohols can be easily obtained by nucleophilic fluoroalkylation of the corresponding aldehydes.
References
.

Inoue M., Sumii Y., Shibata N.
ACS Omega,
2020
.

Böhm H., Banner D., Bendels S., Kansy M., Kuhn B., Müller K., Obst-Sander U., Stahl M.
ChemBioChem,
2004
.

Liu X., Xu C., Wang M., Liu Q.
Chemical Reviews,
2014
.

Ogawa Y., Tokunaga E., Kobayashi O., Hirai K., Shibata N.
iScience,
2020
.

Jamison C.R., Overman L.E.
Accounts of Chemical Research,
2016
.

Zubkov M.O., Kosobokov M.D., Levin V.V., Dilman A.D.
Organic Letters,
2022
.

Zubkov M.O., Kosobokov M.D., Levin V., Kokorekin V.A., Korlyukov A.A., Hu J., Dilman A.D.
Chemical Science,
2020
.

Kosobokov M.D., Zubkov M.O., Levin V.V., Kokorekin V.A., Dilman A.D.
Chemical Communications,
2020
.

Worp B.A., Kosobokov M.D., Levin V.V., Dilman A.D.
Advanced Synthesis and Catalysis,
2020
.

Friese F.W., Studer A.
Angewandte Chemie - International Edition,
2019
.

Zhu L., Wang L., Li B., Fu B., Zhang C., Li W.
Chemical Communications,
2016
.

Wilger D.J., Gesmundo N.J., Nicewicz D.A.
Chemical Science,
2013
.

Mizuta S., Verhoog S., Engle K.M., Khotavivattana T., O’Duill M., Wheelhouse K., Rassias G., Médebielle M., Gouverneur V.
Journal of the American Chemical Society,
2013
.

Barton D.H., McCombie S.W.
Journal of the Chemical Society Perkin Transactions 1,
1975
.

Stache E.E., Ertel A.B., Rovis T., Doyle A.G.
ACS Catalysis,
2018
.

Zemtsov A.A., Lunkov S.S., Levin V.V., Dilman A.D.
European Journal of Organic Chemistry,
2021
.

Togo H., Matsubayashi S., Yamazaki O., Yokoyama M.
Journal of Organic Chemistry,
2000
.

Foti M.C., Ingold K.U.
Journal of Agricultural and Food Chemistry,
2003
.

Davies J., Booth S.G., Essafi S., Dryfe R.A., Leonori D.
Angewandte Chemie - International Edition,
2015
.

Zhang L., Koreeda M.
Journal of the American Chemical Society,
2004
.

Dai X., Li C.
Journal of the American Chemical Society,
2016
.

Vara B.A., Patel N.R., Molander G.A.
ACS Catalysis,
2017
.

Chatgilialoglu C., Ferreri C., Landais Y., Timokhin V.I.
Chemical Reviews,
2018
.

Chen J., Chang R., Lin J., Luo Y., Xu P.
Organic Letters,
2018
.

Paeth M., Tyndall S.B., Chen L., Hong J., Carson W.P., Liu X., Sun X., Liu J., Yang K., Hale E.M., Tierney D.L., Liu B., Cao Z., Cheng M., Goddard W.A., et. al.
Journal of the American Chemical Society,
2019
.

Xie H., Guo J., Wang Y., Wang K., Guo P., Su P., Wang X., Shu X.
Journal of the American Chemical Society,
2020
.
![Phenyl Benzo[ b ]phenothiazine as a Visible Light Photoredox Catalyst for Metal‐Free Atom Transfer Radical Polymerization](/storage/images/resized/bRyGpdm98BkAUYiK1YFNpl5Z7hPu6Gd87gbIeuG3_small_thumb.webp)
Dadashi‐Silab S., Pan X., Matyjaszewski K.
Chemistry - A European Journal,
2017
.

Prakash G.K., Krishnamurti R., Olah G.A.
Journal of the American Chemical Society,
1989
.

Constantin T., Górski B., Tilby M.J., Chelli S., Juliá F., Llaveria J., Gillen K.J., Zipse H., Lakhdar S., Leonori D.
Science,
2022
.

Dong Z., MacMillan D.W.
Nature,
2021
.
Levin V.V., Dilman A.D.
Mendeleev Communications,
2021
.

Hunter L.
Beilstein Journal of Organic Chemistry,
2010
.

Cao D., Chen Z., Lv L., Zeng H., Peng Y., Li C.
iScience,
2020
.

Wang J., Ren Y., Tian X., Ren F., Cheng X., Zhao S.
Synlett,
2018
.

Barton D.H., Crich D.
Tetrahedron Letters,
1985
.

Rackl D., Kais V., Kreitmeier P., Reiser O.
Beilstein Journal of Organic Chemistry,
2014
.
Chenneberg L., Ollivier C.
Chimia,
2016