Keywords
(diarylmethyl)phosphonamidates
1 2-benzoxaphospholes
1 6-conjugate addition
antitumour activity.
cytotoxicity
p-quinone methides
phosphonamidates
Abstract
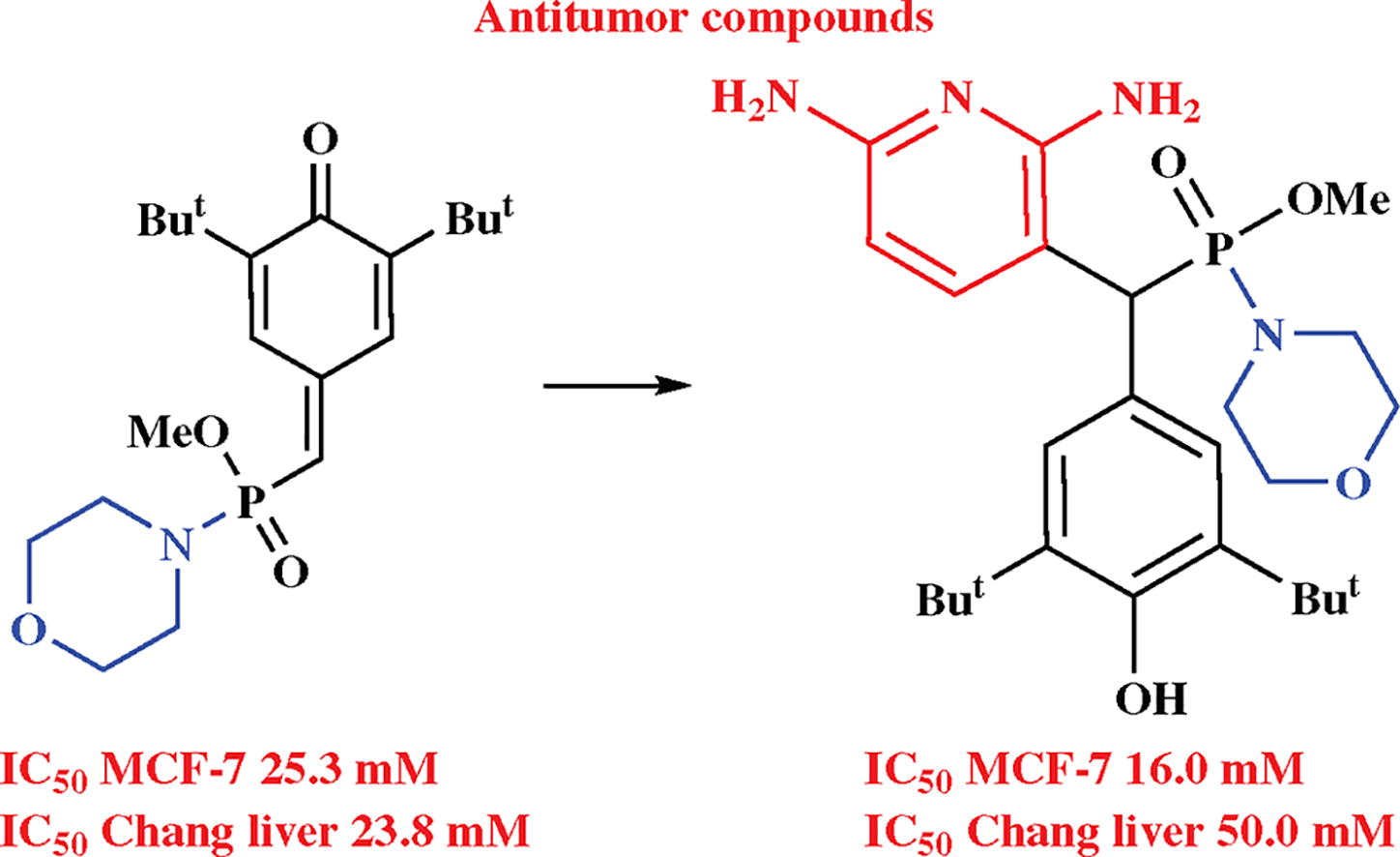
Novel (diarylmethyl)phosphonamidates containing 2,6-di-tert-butylphenol and heterocycle moieties were synthesized by 1,6-conjugate addition of phosphorylated p-quinone methide with morpholine fragment to 2,6-diaminopyridine or 1,3-diaminobenzene. In the case of an acid-catalyzed reaction of p-quinone methide with sesamol, morpholine was cleaved to form 1,2-benzoxaphosphole substituent. Cytotoxic effects of starting compounds and obtained products were evaluated towards human cancer and normal cells.
References
.

Milaeva E.R., Shpakovsky D.B., Gracheva Y.A., Antonenko T.A., Osolodkin D.I., Palyulin V.A., Shevtsov P.N., Neganova M.E., Vinogradova D.V., Shevtsova E.F.
Journal of Organometallic Chemistry,
2015
.

Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F.
Ca-A Cancer Journal for Clinicians,
2021
.

Gibadullina E., Nguyen T.T., Strelnik A., Sapunova A., Voloshina A., Sudakov I., Vyshtakalyuk A., Voronina J., Pudovik M., Burilov A.
European Journal of Medicinal Chemistry,
2019
.
![Reactions of [(3,5-Di-tert-butyl-4-oxocyclohexa-2,5-dien- 1-ylidene)methyl]phosphonates with Phenols](/storage/images/resized/oZgeErrVFhuDksyqFURLvYS1wtVSBWczh001igGo_small_thumb.webp)
Reactions of [(3,5-Di-tert-butyl-4-oxocyclohexa-2,5-dien- 1-ylidene)methyl]phosphonates with Phenols
Gibadullina E.M., Shaekhov T.R., Voronina Y.K., Pudovik M.A., Burilov A.R.
Russian Journal of Organic Chemistry,
2018
.

Jiang X., Hu C., Ferchen K., Nie J., Cui X., Chen C., Cheng L., Zuo Z., Seibel W., He C., Tang Y., Skibbe J.R., Wunderlich M., Reinhold W.C., Dong L., et. al.
Nature Communications,
2017
.

Kuehl P.J., Stratton S.P., Powell M.B., Myrdal P.B.
International Journal of Pharmaceutics,
2009
.
![Benzo[d]-1,2-oxaphospholes as Precursors of Stabilized C-Centered Radicals](/storage/images/resized/iLiQsFqFaSEx6chlGQ5fbAwF6VYU3WWa08hkss0g_small_thumb.webp)
Pérez-Prieto J., Galian R.E., Miranda M.A., Catalina F., Martín-Vargas N., López-Ortiz F.
Organic Letters,
2004
.

Demkowicz S., Rachon J., Daśko M., Kozak W.
RSC Advances,
2016
.

Sawa M., Kiyoi T., Kurokawa K., Kumihara H., Yamamoto M., Miyasaka T., Ito Y., Hirayama R., Inoue T., Kirii Y., Nishiwaki E., Ohmoto H., Maeda Y., Ishibushi E., Inoue Y., et. al.
Journal of Medicinal Chemistry,
2002
.

Molleti N., Kang J.Y.
Organic Letters,
2017
.

Lentini N.A., Foust B.J., Hsiao C.C., Wiemer A.J., Wiemer D.F.
Journal of Medicinal Chemistry,
2018
.

Xu S., Xie J., Liu Y., Xu W., Tang K., Xiong B., Wong W.
Journal of Organic Chemistry,
2021
.

Parra A., Tortosa M.
ChemCatChem,
2015
.

Fredriksen K.A., Amedjkouh M.
European Journal of Organic Chemistry,
2015
.

Zhang B., Liu L., Mao S., Zhou M., Wang H., Li L.
European Journal of Organic Chemistry,
2019
.

Tzara A., Xanthopoulos D., Kourounakis A.P.
ChemMedChem,
2020
.

Lima C.G., Pauli F.P., Costa D.C., de Souza A.S., Forezi L.S., Ferreira V.F., de Carvalho da Silva F.
European Journal of Organic Chemistry,
2020
.

Winter M., Schütz R., Eitzinger A., Ofial A.R., Waser M.
European Journal of Organic Chemistry,
2020
.

Shirsath S.R., More D.A., Muthukrishnan M.
Chemistry - An Asian Journal,
2022
.

Eitzinger A., Winter M., Schörgenhumer J., Waser M.
Chemical Communications,
2020
.

Ghatak S., Vyas A., Misra S., O’Brien P., Zambre A., Fresco V.M., Markwald R.R., Swamy K.V., Afrasiabi Z., Choudhury A., Khetmalas M., Padhye S.
Bioorganic and Medicinal Chemistry Letters,
2014
.

Liu H., Wu B., Ge Y., Huang J., Song S., Wang C., Yao J., Liu K., Li Y., Li Y., Ma X.
Bioorganic and Medicinal Chemistry,
2017
.
Tham P.T., Chinh P.T., Tuyen N.V., Bang D.N., Van D.T., Kien V.T., Thanh H.T., Quynh D.H., Cuong V.D., Thanh N.H., Pérez-Encabo A.
Mendeleev Communications,
2021
.
Kibardina L.K., Trifonov A.V., Dobrynin A.B., Pudovik M.A., Burilov A.R., Voloshina A.D., Strelnik A.G., Gazizov A.S.
Mendeleev Communications,
2021
.
Chugunova E.A., Smolobochkin A.V., Gazizov A.S., Burilov A.R., Voloshina A.D., Lyubina A.P., Amerhanova S.K., Melnikova A.A., Tulesinova A.I., Akylbekov N.I., Akhatayev N.A., Syakaev V.V.
Mendeleev Communications,
2021