Keywords
2
3-dihydroxynaphthazarin
4-naphthoquinone
5
8-dihydroxy-1
hydrolysis
naphthazarin
organic azides
sea urchin pigments.
spinazarin
Abstract
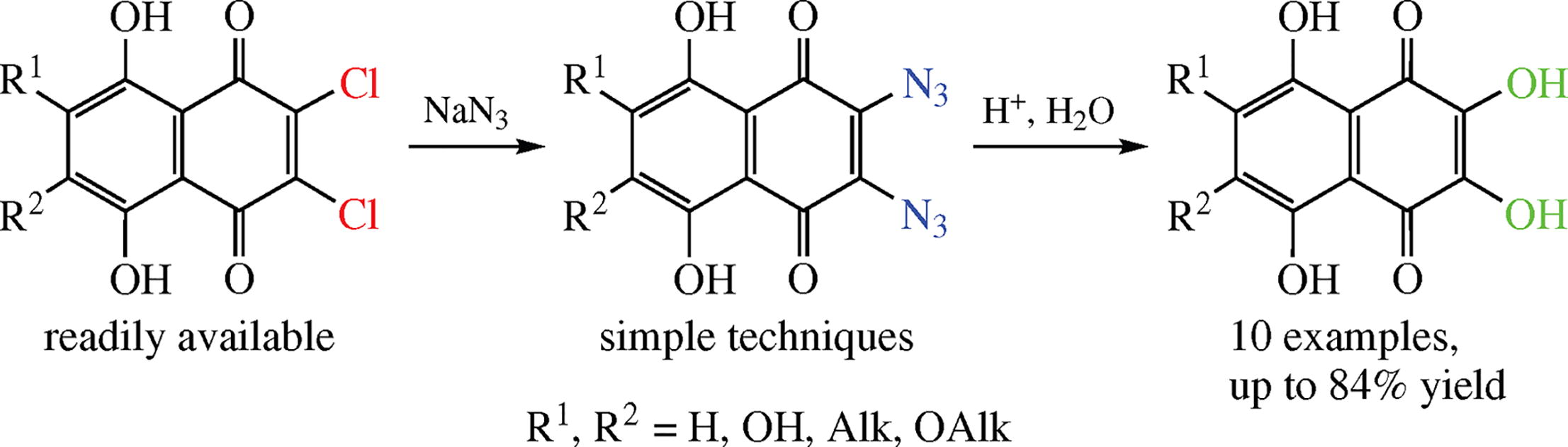
A short simple synthesis of spinazarins (2,3-dihydroxy-naphthazarins or 2,3,5,8-tetrahydroxy-1,4-naphtho-quinones) from available 2,3-dichloronaphthazarin derivatives involves replacement of chlorine atoms with azido groups followed by their acidic hydrolysis. The procedure can be used for the preparative synthesis of natural biologically active spinazarins and their analogues.
References
.

Bräse S., Gil C., Knepper K., Zimmermann V.
Angewandte Chemie - International Edition,
2005
.

Dyshlovoy S.A., Pelageev D.N., Jakob L.S., Borisova K.L., Hauschild J., Busenbender T., Kaune M., Khmelevskaya E.A., Graefen M., Bokemeyer C., Anufriev V.P., von Amsberg G.
Pharmaceuticals,
2021
.

Salmon-Chemin L., Buisine E., Yardley V., Kohler S., Debreu M., Landry V., Sergheraert C., Croft S.L., Krauth-Siegel R.L., Davioud-Charvet E.
Journal of Medicinal Chemistry,
2001
.

Dyshlovoy S.A., Pelageev D.N., Hauschild J., Sabutskii Y.E., Khmelevskaya E.A., Krisp C., Kaune M., Venz S., Borisova K.L., Busenbender T., Denisenko V.A., Schlüter H., Bokemeyer C., Graefen M., Polonik S.G., et. al.
Marine Drugs,
2020
.

Wellington K.W.
RSC Advances,
2015
.

Chapyshev S.
Molecules,
2015
.

Yoon C., Kim H., Mishchenko N., Vasileva E., Fedoreyev S., Stonik V., Han J.
Marine Drugs,
2018
.

Fedoreyev S., Krylova N., Mishchenko N., Vasileva E., Pislyagin E., Iunikhina O., Lavrov V., Svitich O., Ebralidze L., Leonova G.
Marine Drugs,
2018
.

Mischenko N.P., Fedoreyev S.A., Pokhilo N.D., Anufriev V.P., Denisenko V.A., Glazunov V.P.
Journal of Natural Products,
2005
.

Pokhilo N.D., Shuvalova M.I., Lebedko M.V., Sopelnyak G.I., Yakubovskaya A.Y., Mischenko N.P., Fedoreyev S.A., Anufriev V.P.
Journal of Natural Products,
2006
.

Vasileva E.A., Mishchenko N.P., Fedoreyev S.A.
Chemistry and Biodiversity,
2017
.

Novikov V.L., Balaneva N.N., Shestak O.P., Anufriev V.P., Glazunov V.P.
Russian Chemical Bulletin,
2016
.

Yakubovskaya A.Y., Pokhilo N.D., Mishchenko N.P., Anufriev V.F.
Russian Chemical Bulletin,
2007
.

Polonik S.G., Polonik N.S., Denisenko V.A., Moiseenko O.P., Anufriev V.F.
Russian Journal of Organic Chemistry,
2009
.

Singh I., Moore R.E., Chang C.W., Scheuer P.J.
Journal of the American Chemical Society,
1965
.

Moore H.W., Perri S.T.
Journal of Organic Chemistry,
1988
.

Huot R., Brassard P.
Canadian Journal of Chemistry,
1974
.

Anderson H.A., Smith J., Thomson R.H.
Journal of the Chemical Society (Resumed),
1965
.

Moore H.W.
Chemical Society Reviews,
1973
.

Shikov A.N., Pozharitskaya O.N., Krishtopina A.S., Makarov V.G.
Phytochemistry Reviews,
2018
.

Kochergina T.Y., Anufriev V.F.
Chemistry of Natural Compounds,
2011
.

Pokhilo N.D., Kiseleva M.I., Anufriev V.F.
Pharmaceutical Chemistry Journal,
2011
.

Sánchez-Calvo J.M., Barbero G.R., Guerrero-Vásquez G., Durán A.G., Macías M., Rodríguez-Iglesias M.A., Molinillo J.M., Macías F.A.
Medicinal Chemistry Research,
2016
.

Anufriev V.P., Polonik S.G., Pokhilo N.D., Balanyova N.N.
Russian Chemical Bulletin,
2003
.

Weygand F., Vogelbach K., Zimmermann K.
Chemische Berichte,
1947
.

Anufriev V.P., Novikov V.L.
Tetrahedron Letters,
1995
.

Anufriey V.P., Novikov V.L., Maximov O.B., Elyakov G.B., Levitsky D.O., Lebedev A.V., Sadretdinov S.M., Shvilkin A.V., Afonskaya N.I., Ruda M.Y., Cherpachenko N.M.
Bioorganic and Medicinal Chemistry Letters,
1998
.

Mishchenko N.P., Vasileva E.A., Fedoreyev S.A.
Tetrahedron Letters,
2014
.

Melman G.I., Borisova K.L., Pokhilo N.D., Makhankov V.V., Anufriev V.P.
Tetrahedron Letters,
2016
.

Polonik N.S., Polonik S.G.
Tetrahedron Letters,
2016
.
FUTURO D.O., FERREIRA P.G., NICOLETTI C.D., BORBA-SANTOS L.P., SILVA F.C., ROZENTAL S., FERREIRA V.F.
Anais da Academia Brasileira de Ciencias,
2018
.

Zhou D., Qin L., Zhu B., Wang X., Tan H., Yang J., Li D., Dong X., Wu H., Sun L., Li X., Murata Y.
Food Chemistry,
2011
.

El Hage S., Ane M., Stigliani J., Marjorie M., Vial H., Baziard-Mouysset G., Payard M.
European Journal of Medicinal Chemistry,
2009
.

Pokhilo N.D., Yakubovskaya A.Y., Denisenko V.A., Anufriev V.P.
Tetrahedron Letters,
2006
.

Polonik N., Polonik S., Denisenko V., Moiseenko O.
Synthesis,
2011
.

Anufriev V., Pelageev D.
Synthesis,
2016
.

Polonik S., Sabutskii Y., Denisenko V.
Synthesis,
2018
.
.

Singh I., Moore R.E., Chang C.W., Ogata R.T., Scheuer P.J.
Tetrahedron,
1968
.

Aminin D., Polonik S.
Chemical and Pharmaceutical Bulletin,
2019