Keywords
1-oxa-9-azaspiro[5.5]undecanes
carbonic anhydrases
chemical diversity
druglikeness.
spiro compounds
sulfamide zinc binding group
Abstract
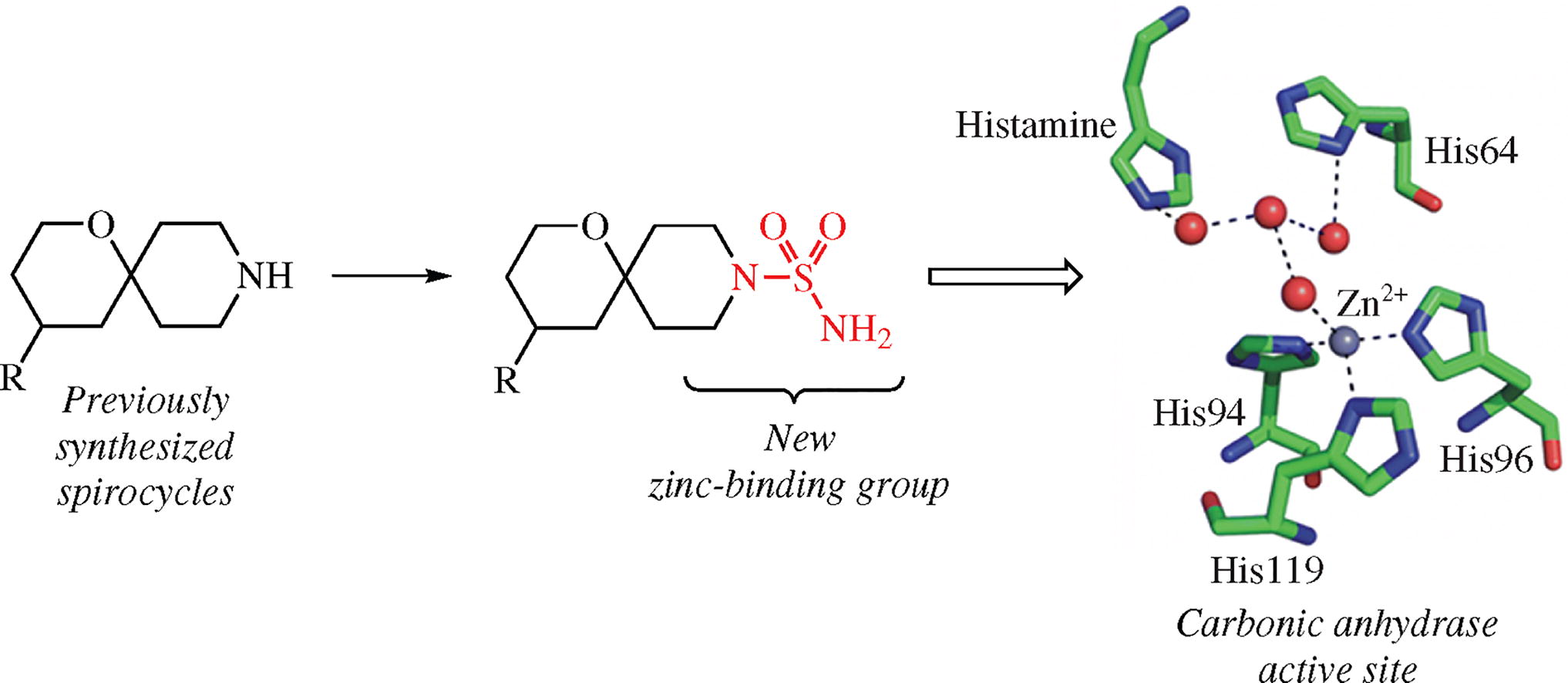
A fundamentally novel type of molecular probes for the recognition by carbonic anhydrase zinc enzymes which constitute promising target family in diverse therapeutic areas has been designed and synthesized. To the best of our knowledge, these molecular tools of 1-oxa-9-azaspiro[5.5]-undecane-9-sulfonamide chemotype for the first time combine in their structure diversely substituted spirocyclic piperidines and an aminosulfamoyl moiety.
References
.

Hiesinger K., Dar’in D., Proschak E., Krasavin M.
Journal of Medicinal Chemistry,
2020
.

Lipinski C.A., Lombardo F., Dominy B.W., Feeney P.J.
Advanced Drug Delivery Reviews,
2001
.

Krasavin M., Lukin A., Bagnyukova D., Zhurilo N., Zahanich I., Zozulya S., Ihalainen J., Forsberg M.M., Lehtonen M., Rautio J., Moore D., Tikhonova I.G.
Bioorganic and Medicinal Chemistry,
2016
.

Lukin A., Chudinov M., Vedekhina T., Rogacheva E., Kraeva L., Bakulina O., Krasavin M.
Molecules,
2022
.

Krasavin M., Lukin A., Bagnyukova D., Zhurilo N., Zahanich I., Zozulya S.
Journal of Enzyme Inhibition and Medicinal Chemistry,
2016
.

Supuran C.T.
Journal of Enzyme Inhibition and Medicinal Chemistry,
2012
.
Paramonova P., Sharonova T., Kalinin S., Chupakhin E., Bunev A., Krasavin M.
Mendeleev Communications,
2022
.

Scozzafava A., Supuran C.T.
Sub-Cellular Biochemistry,
2013
.

Pastorekova S., Parkkila S., Pastorek J., Supuran C.T.
Journal of Enzyme Inhibition and Medicinal Chemistry,
2004
.

Carta F., Vullo D., Osman S.M., AlOthman Z., Supuran C.T.
Bioorganic and Medicinal Chemistry,
2017
.

McDonald P.C., Chia S., Bedard P.L., Chu Q., Lyle M., Tang L., Singh M., Zhang Z., Supuran C.T., Renouf D.J., Dedhar S.
American Journal of Clinical Oncology: Cancer Clinical Trials,
2020
.

Alterio V., Di Fiore A., D’Ambrosio K., Supuran C.T., De Simone G.
Chemical Reviews,
2012
.

Wongboonsin J., Thongprayoon C., Bathini T., Ungprasert P., Aeddula N., Mao M., Cheungpasitporn W.
Journal of Clinical Medicine,
2019
.

Ciccone L., Cerri C., Nencetti S., Orlandini E.
Molecules,
2021
.

Klinger A.L., McComsey D.F., Smith-Swintosky V., Shank R.P., Maryanoff B.E.
Journal of Medicinal Chemistry,
2006
.

D'Ambrosio K., Smaine F., Carta F., De Simone G., Winum J., Supuran C.T.
Journal of Medicinal Chemistry,
2012
.

Assi R., Kantarjian H.M., Kadia T.M., Pemmaraju N., Jabbour E., Jain N., Daver N., Estrov Z., Uehara T., Owa T., Cortes J.E., Borthakur G.
Cancer,
2018
.

Supuran C.T.
Expert Review of Neurotherapeutics,
2015
.

Buzás G.M., Supuran C.T.
Journal of Enzyme Inhibition and Medicinal Chemistry,
2015
.

Swenson E.R.
Sub-Cellular Biochemistry,
2013
.
Ward C., Meehan J., Gray M.E., Murray A.F., Argyle D.J., Kunkler I.H., Langdon S.P.
Exploration of Targeted Anti-tumor Therapy,
2020