Keywords
acyl fluorides
bicyclo[3.1.0]hex-2-ene-6-carboxylic acids
Diels–Alder reaction
epoxidation
Meinwald rearrangement
nitrostyrene
norbornadiene
norbornene
organofluorine compounds.
Abstract
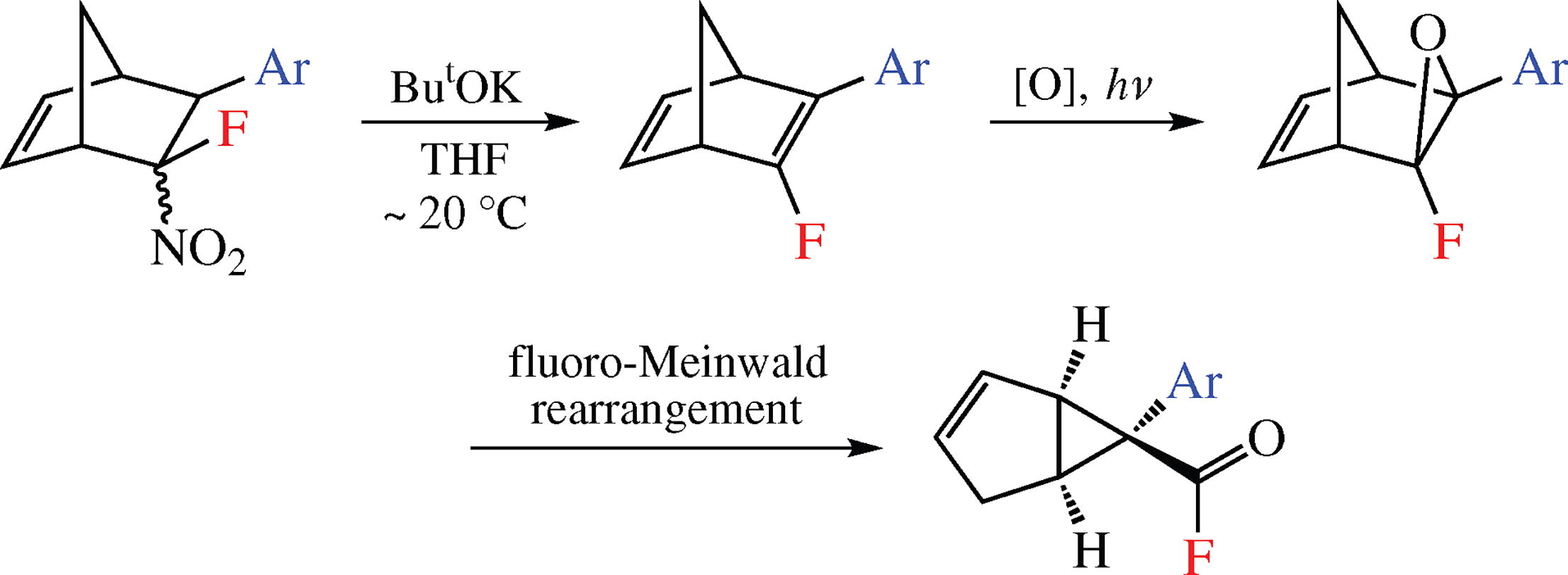
Novel fluorinated norbornadienes were synthesized in up to 95% yield by the base-induced elimination of HNO2 from 5-fluoro-5-nitro-6-arylbicyclo[2.2.1]hept-2-enes prepared, in turn, by the Diels-Alder reaction of β-fluoro-β-nitrostyrenes with cyclopentadiene. The subsequent epoxidation initiated the Meinwald type rearrangement affording 6-aryl-bicyclo[3.1.0]hex-2-ene-6-carboxylic acid fluorides as individual (1SR,5RS,6RS)-diastereomers. The transformation is the first example of fluoro-Meinwald rearrangement to form the corresponding acyl fluorides.
References
.

Krause L., Herbst-Irmer R., Sheldrick G.M., Stalke D.
Journal of Applied Crystallography,
2015
.

Flid V.R., Gringolts M.L., Shamsiev R.S., Finkelshtein E.S.
Russian Chemical Reviews,
2018
.

Motornov V.A., Muzalevskiy V.M., Tabolin A.A., Novikov R.A., Nelyubina Y.V., Nenajdenko V.G., Ioffe S.L.
Journal of Organic Chemistry,
2017
.

Politanskaya L.V., Selivanova G.A., Panteleeva E.V., Tretyakov E.V., Platonov V.E., Nikul’shin P.V., Vinogradov A.S., Zonov Y.V., Karpov V.M., Mezhenkova T.V., Vasilyev A.V., Koldobskii A.B., Shilova O.S., Morozova S.M., Burgart Y.V., et. al.
Russian Chemical Reviews,
2019
.

Finkelshtein E.S., Bermeshev M.V., Gringolts M.L., Starannikova L.E., Yampolskii Y.P.
Russian Chemical Reviews,
2011
.

Ponomarev S.A., Larkovich R.V., Aldoshin A.S., Tabolin A.A., Ioffe S.L., Groß J., Opatz T., Nenajdenko V.G.
Beilstein Journal of Organic Chemistry,
2021
.

Mamedova V.L., Khikmatova G.Z.
Chemistry of Heterocyclic Compounds,
2017
.
![Peracid Reactions. III.1 The Oxidation of Bicyclo [2.2.1]heptadiene2](/storage/images/resized/iLiQsFqFaSEx6chlGQ5fbAwF6VYU3WWa08hkss0g_small_thumb.webp)
Meinwald J., Labana S.S., Chadha M.S.
Journal of the American Chemical Society,
1963
.
Shastin A.V., Muzalevsky V.M., Balenkova E.S., Nenajdenko V.G.
Mendeleev Communications,
2006
.

Dzhemilev U.M., Khusnutdinov R.I., Tolstikov G.A.
Russian Chemical Reviews,
1987
.

Evstigneeva E.M., Flid V.R.
Russian Chemical Bulletin,
2008
.

Orrego-Hernández J., Dreos A., Moth-Poulsen K.
Accounts of Chemical Research,
2020
.

Khan R., Chen J., Fan B.
Advanced Synthesis and Catalysis,
2020
.

Brown H.C., Liu K.
Journal of the American Chemical Society,
1970
.

Bren' V.A., Dubonosov A.D., Minkin V.I., Chernoivanov V.A.
Russian Chemical Reviews,
1991
.

Polishchuk V.R., Mysov E.I., Stankevitch I.V., Chistyakov A.L., Potechin K.A., Struchkov Y.T.
Journal of Fluorine Chemistry,
1993
.

Dubonosov A.D., Bren V.A., Chernoivanov V.A.
Russian Chemical Reviews,
2002
.

Shastin A.V., Nenajdenko V.G., Muzalevskiy V.M., Balenkova E.S., Fröhlich R., Haufe G.
Tetrahedron,
2008
.

Nişancı B., Dalkılıç E., Güney M., Daştan A.
Beilstein Journal of Organic Chemistry,
2009