Keywords
1
2-a][1
3
5-triazine
5]triazine
aziridine
biocompatibility
cyanuric chloride
cytotoxic activity.
imidazo[1
interaction with DNA
physicochemical properties
Abstract
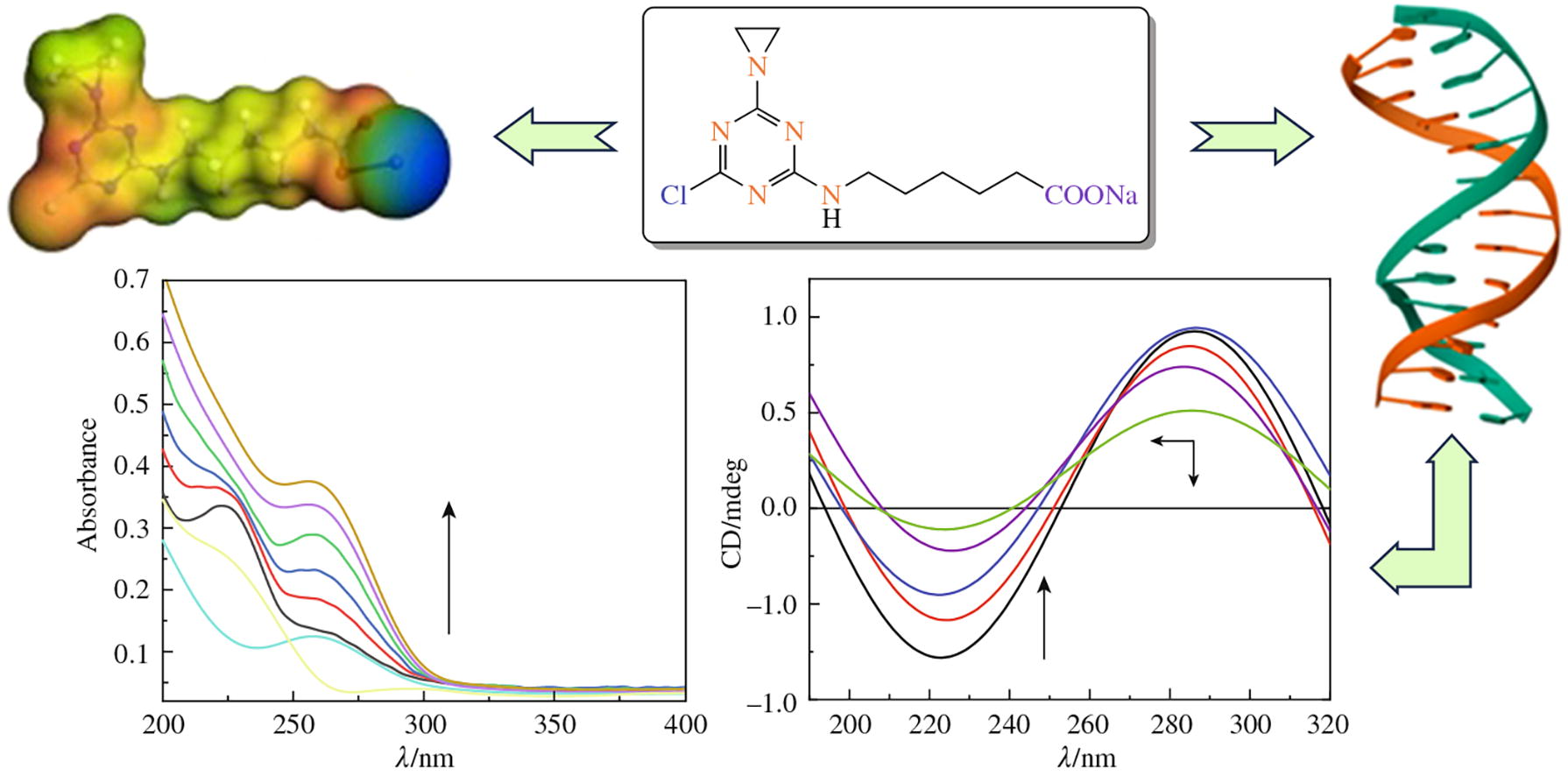
6-{[4-(Aziridin-1-yl)-6-chloro-1,3,5-triazin-2-yl]amino}-hexanoic acid methyl ester and sodium salt were synthesized from cyanuric chloride. The sodium salt would interact with free radicals of 2,2-diphenyl-1-picrylhydrazyl (DPPH) and inhibit hemolysis induced by Radachlorin. Both compounds interact with DNA with Kbin values of 1.09 × 107 and 0.99 × 107 dm3 mol−1, respectively; they also demonstrate cytotoxic activity against human cancer cell lines.
References
.

Cascioferro S., Parrino B., Spanò V., Carbone A., Montalbano A., Barraja P., Diana P., Cirrincione G.
European Journal of Medicinal Chemistry,
2017
.

Rehman S.U., Sarwar T., Husain M.A., Ishqi H.M., Tabish M.
Archives of Biochemistry and Biophysics,
2015
.

Sathisaran I., Dalvi S.
Pharmaceutics,
2018
.

Eropkin M.Y., Melenevskaya E.Y., Nasonova K.V., Bryazzhikova T.S., Eropkina E.M., Danilenko D.M., Kiselev O.I.
Pharmaceutical Chemistry Journal,
2013
.

Maliszewski D., Drozdowska D.
Pharmaceuticals,
2022
.

Beaufils F., Cmiljanovic N., Cmiljanovic V., Bohnacker T., Melone A., Marone R., Jackson E., Zhang X., Sele A., Borsari C., Mestan J., Hebeisen P., Hillmann P., Giese B., Zvelebil M., et. al.
Journal of Medicinal Chemistry,
2017
.
![Discovery and Preclinical Characterization of 5-[4,6-Bis({3-oxa-8-azabicyclo[3.2.1]octan-8-yl})-1,3,5-triazin-2-yl]-4-(difluoromethyl)pyridin-2-amine (PQR620), a Highly Potent and Selective mTORC1/2 Inhibitor for Cancer and Neurological Disorders](/storage/images/resized/iLiQsFqFaSEx6chlGQ5fbAwF6VYU3WWa08hkss0g_small_thumb.webp)
Rageot D., Bohnacker T., Melone A., Langlois J., Borsari C., Hillmann P., Sele A.M., Beaufils F., Zvelebil M., Hebeisen P., Löscher W., Burke J., Fabbro D., Wymann M.P.
Journal of Medicinal Chemistry,
2018
.

Prasher P., Sharma M., Aljabali A.A., Gupta G., Negi P., Kapoor D.N., Singh I., Zacconi F.C., Jesus Andreoli Pinto T., Silva M.W., Bakshi H.A., Chellappan D.K., Tambuwala M.M., Dua K.
Drug Development Research,
2020
.

Majeed Ganai A., Khan Pathan T., Hampannavar G.A., Pawar C., Obakachi V.A., Kushwaha B., Deshwar Kushwaha N., Karpoormath R.
ChemistrySelect,
2021
.

Singh S., Mandal M.K., Masih A., Saha A., Ghosh S.K., Bhat H.R., Singh U.P.
Archiv der Pharmazie,
2021
.

Wong J.R., Morton L.M., Tucker M.A., Abramson D.H., Seddon J.M., Sampson J.N., Kleinerman R.A.
Journal of Clinical Oncology,
2014
.

Krętowski R., Drozdowska D., Kolesińska B., Kamiński Z., Frączyk J., Cechowska-Pasko M.
Investigational New Drugs,
2019
.

Srivastava J.K., Pillai G.G., Bhat H.R., Verma A., Singh U.P.
Scientific Reports,
2017
.

Lim F.P., Dolzhenko A.V.
European Journal of Medicinal Chemistry,
2014
.

Venkataraman K., Wagle D.R.
Tetrahedron Letters,
1979
.

Singla P., Luxami V., Paul K.
European Journal of Medicinal Chemistry,
2015
.

Pizarro A.M., Sadler P.J.
Biochimie,
2009
.

Hu J., Zhang Y., Tang N., Lu Y., Guo P., Huang Z.
Bioorganic and Medicinal Chemistry,
2021
.

Hiebel M., Suzenet F.
Progress in Heterocyclic Chemistry,
2021
.
Mikolaichuk O.V., Shemchuk O.S., Protas A.V., Popova E.A., Ostrovskii V.A., Maystrenko D.N., Molchanov O.E., Sharoyko V.V., Semenov K.N.
Mendeleev Communications,
2023