Keywords
2-fluorobenzoyl substituent.
anticonvulsant
barbiturates
enantiomer
GABAA receptor
molecular docking
γ-aminobutyric acid
Abstract
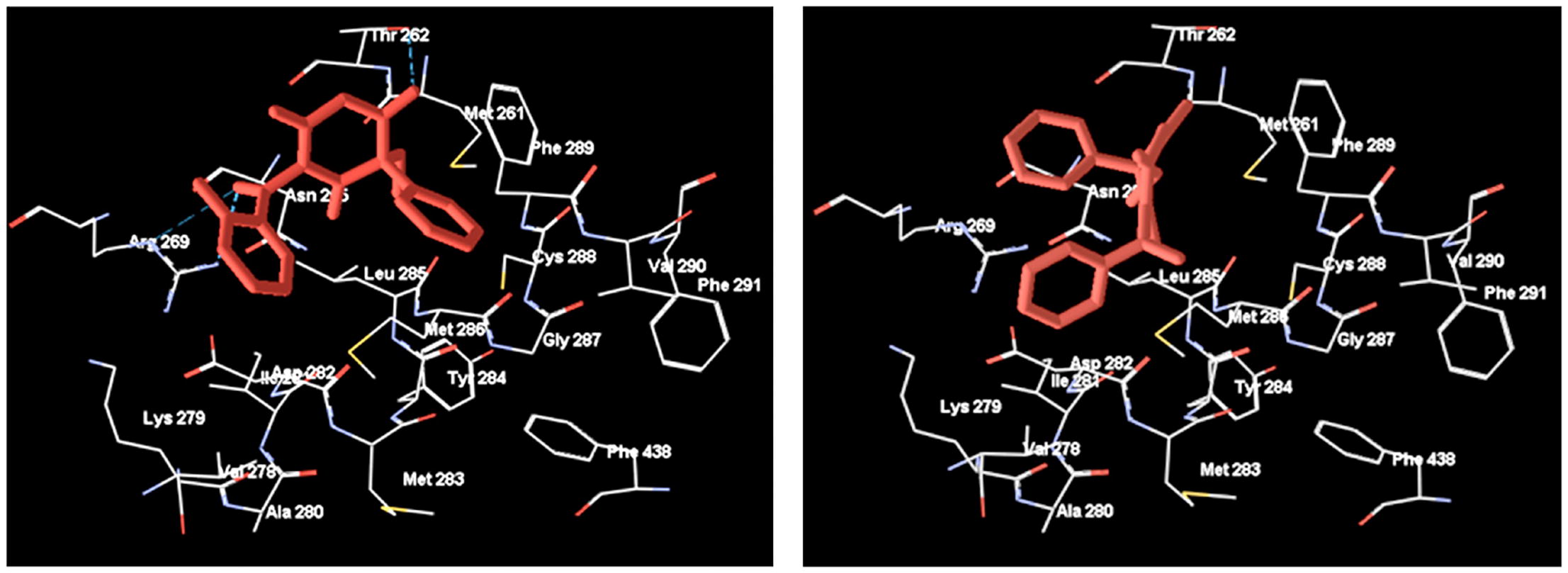
An original phenobarbital anticonvulsant Halonal, 5-ethyl-1-(2-fluorobenzoyl)-5-phenylpyrimidine-2,4,6(1H,3H,5H)-trione, stimulated the cellular immune and the humoral response in long-term alcoholized male (CBAxC57Bl/6) F1 mice to the level of healthy animals. Voltammetry was found to be suitable for determination of Halonal R/S-enantiomeric ratio, which was exemplified on the authentic sample with the R/S-composition of 40:60. Molecular docking (Schrodinger program, Glide) showed that Halonal behaved as a benzonal derivative interacting with GABAAR via the BARB binding site, with S-Halonal having higher similarity score than its R-enantiomer because of a different orientation of the 2-fluorobenzoyl substituent.
References
.

Amundarain M.J., Viso J.F., Zamarreño F., Giorgetti A., Costabel M.
Journal of Biomolecular Structure and Dynamics,
2018
.

De Santis S., Cosa-Linan A., Garcia-Hernandez R., Dmytrenko L., Vargova L., Vorisek I., Stopponi S., Bach P., Kirsch P., Kiefer F., Ciccocioppo R., Sykova E., Moratal D., Sommer W.H., Canals S., et. al.
Science advances,
2020
.

Olsen R.W., Liang J.
Molecular Brain,
2017
.

Tan K.R., Baur R., Charon S., Goeldner M., Sigel E.
Journal of Neurochemistry,
2009
.

Koob G.F., Volkow N.D.
The Lancet Psychiatry,
2016
.

Di L., Whitney‐Pickett C., Umland J.P., Zhang H., Zhang X., Gebhard D.F., Lai Y., Federico J.J., Davidson R.E., Smith R., Reyner E.L., Lee C., Feng B., Rotter C., Varma M.V., et. al.
Journal of Pharmaceutical Sciences,
2011
.

Hanson S.M., Czajkowski C.
Journal of Neuroscience,
2008
.

Friesner R.A., Murphy R.B., Repasky M.P., Frye L.L., Greenwood J.R., Halgren T.A., Sanschagrin P.C., Mainz D.T.
Journal of Medicinal Chemistry,
2006
.

Halgren T.A., Murphy R.B., Friesner R.A., Beard H.S., Frye L.L., Pollard W.T., Banks J.L.
Journal of Medicinal Chemistry,
2004
.

Madhavi Sastry G., Adzhigirey M., Day T., Annabhimoju R., Sherman W.
Journal of Computer-Aided Molecular Design,
2013
.

Mezentseva O., Slepchenko G., Filimonov V., Mikheeva E., Arbit G.
Electroanalysis,
2019
.

Bergman I., Bishop K., Tu Q., Frech W., Ã
kerblom S., Nilsson M.
PLoS ONE,
2013
.

Shushpanova T.V., Bokhan N.A., Stankevich K.S., Novozheeva T.P., Mandel’ A.I., Schastnyi E.D., Kisel’ N.I., Shushpanova O.V., Udut V.V., Safronov S.M., Boev R.S., Knyazeva E.M.
Pharmaceutical Chemistry Journal,
2021
.

Miller P.S., Aricescu A.R.
Nature,
2014
.
Buhr A., Schaerer M.T., Baur R., Sigel E.
Molecular Pharmacology,
1997
.

Bialer M.
Epilepsia,
2012
.

Pflanz N.C., Daszkowski A.W., Cornelison G.L., Trudell J.R., Mihic S.J.
Journal of Biological Chemistry,
2018
.

Ceccato A., Boulanger B., Chiap P., Hubert P., Crommen J.
Journal of Chromatography A,
1998
.

Amundarain M.J., Ribeiro R.P., Costabel M.D., Giorgetti A.
Future Medicinal Chemistry,
2019