Keywords
C–C bond formation
cyanoacetates
Knoevenagel condensation
manganese catalysis
reduction
syngas
Abstract
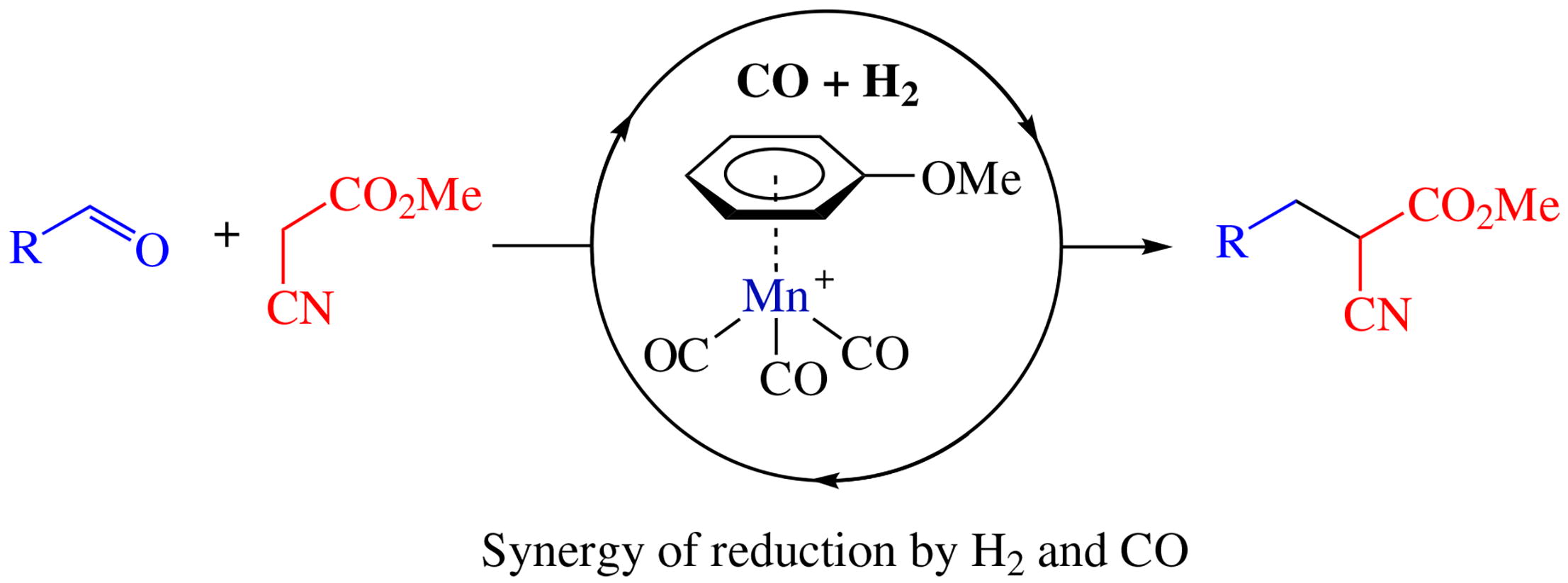
Arene manganese π-complex was applied as a catalyst for the reductive Knoevenagel condensation. Syngas was used as a selective synergistic reducing agent providing the possibility to use 3d metal-based catalyst in such process. Demonstrated substrate scope indicates a high potential for this reaction in a medicinal chemistry.
References
1.

Verde-Sesto E., Merino E., Rangel-Rangel E., Corma A., Iglesias M., Sánchez F.
ACS Sustainable Chemistry and Engineering,
2016
2.

Gandeepan P., Müller T., Zell D., Cera G., Warratz S., Ackermann L.
Chemical Reviews,
2018
3.

Afanasyev O.I., Zarochintsev A., Petrushina T., Cherkasova A., Denisov G., Cherkashchenko I., Chusova O., Jinho O., Man-Seog C., Usanov D.L., Semenov S.E., Chusov D.
European Journal of Organic Chemistry,
2018
4.

Podyacheva E., Afanasyev O.I., Ostrovskii V.S., Chusov D.
ACS Catalysis,
2022
5.

Yang W., Chernyshov I.Y., van Schendel R.K., Weber M., Müller C., Filonenko G.A., Pidko E.A.
Nature Communications,
2021
6.

Muratov K., Kuchuk E., Vellalath S., Afanasyev O.I., Moskovets A.P., Denisov G., Chusov D.
Organic and Biomolecular Chemistry,
2018
7.

Kolesnikov P.N., Usanov D.L., Barablina E.A., Maleev V.I., Chusov D.
Organic Letters,
2014
8.

Yagafarov N.Z., Usanov D.L., Moskovets A.P., Kagramanov N.D., Maleev V.I., Chusov D.
ChemCatChem,
2015
9.

Denmark S.E., Ibrahim M.Y., Ambrosi A.
ACS Catalysis,
2016
10.

Huang M., Li Y., Li Y., Liu J., Shu S., Liu Y., Ke Z.
Chemical Communications,
2019
11.

Valyaev D.A., Lavigne G., Lugan N.
Coordination Chemistry Reviews,
2016
12.

Design of Manganese Phenol Pi‐complexes as Shvo‐type Catalysts for Transfer Hydrogenation of Ketones
Shvydkiy N.V., Vyhivskyi O., Nelyubina Y.V., Perekalin D.S.
ChemCatChem,
2019
13.

Oh M., Reingold J.A., Carpenter G.B., Sweigart D.A.
Coordination Chemistry Reviews,
2004
14.

Fillion E., Fishlock D.
Organic Letters,
2003
15.

Peraka S., Hussain A., Ramachary D.B.
Journal of Organic Chemistry,
2018
16.

Podyacheva E., Afanasyev O.I., Vasilyev D.V., Chusov D.
ACS Catalysis,
2022
17.

Runikhina S., Eremin D., Chusov D.
Chemistry - A European Journal,
2021
18.

Goettmann F., Grosso D., Mercier F., Mathey F., Sanchez C.
Chemical Communications,
2004
19.

Ramachary D.B., Venkaiah C., Reddy Y.V., Kishor M.
Organic and Biomolecular Chemistry,
2009
20.

Ramachary D.B., Venkaiah C., Reddy Y.V.
Organic and Biomolecular Chemistry,
2014
21.

Li P., Yu Y., Liu H., Cao C., Song W.
Nanoscale,
2014
22.

Guyon C., Duclos M., Sutter M., Métay E., Lemaire M.
Organic and Biomolecular Chemistry,
2015
23.

Wang H., Wang Y., Jia A., Wang C., Wu L., Yang Y., Wang Y.
Catalysis Science and Technology,
2017
24.

Jiang G., Liu M., Fang D., Tan P., Huang M., Zhou T., Jiang Z., Xu Z., Wang Z.
RSC Advances,
2018
25.

Bobrova A.Y., Novikov M.A., Tomilov Y.V.
Organic and Biomolecular Chemistry,
2021
26.

Abe F., Hayashi T., Tanaka M.
Chemistry Letters,
1990
27.

Hrubowchak D.M., Smith F.X.
Tetrahedron Letters,
1983
28.

Tóth G., Kövér K.E.
Synthetic Communications,
1995
29.

Huang X., Xie L.
Synthetic Communications,
1986
30.

Li P., Liu H., Yu Y., Cao C., Song W.
Chemistry - An Asian Journal,
2013
31.

Yang Y., Lu Z.
Chinese Journal of Chemistry,
2014
32.

Patankar S.C., Dodiya S.K., Yadav G.D.
Journal of Molecular Catalysis A Chemical,
2015
33.

Andrianova A.A., Maslova Y.D., Novikov M.A., Semenov S.E., Nefedov O.M.
Journal of Fluorine Chemistry,
2018
34.
![A new reversible transformation of oxindolylidene derivatives of imidazothiazolotriazine into 3-[(imidazotriazin-3-yl)thio]-2-oxoquinoline-4-carboxylates](/storage/images/resized/leiAYcRDGTSl5B1eCnwpSGqmDEUEfDPPoYisFGhT_small_thumb.webp)
Izmest'ev A.N., Kravchenko A.N., Gazieva G.A.
Organic and Biomolecular Chemistry,
2023
35.

Mudhar H., Witty A.
Tetrahedron Letters,
2010
36.

Li J., Liu Q., Xing R.G., Shen X.X., Liu Z.G., Zhou B.
Chinese Chemical Letters,
2009
37.

Baidossi M., Joshi A.V., Mukhopadhyay S., Sasson Y.
Tetrahedron Letters,
2005
38.

Motokura K., Fujita N., Mori K., Mizugaki T., Ebitani K., Kaneda K.
Tetrahedron Letters,
2005
39.

Ramachary D.B., Kishor M., Ramakumar K.
Tetrahedron Letters,
2006
40.

Linton M., Gonzalez J., Li H., Tatlock J., Jewell T., Johnson S., Drowns M., Patel L., Blazel J., Ornelas M.
Synthesis,
2010
41.
![2-Phenyl-2,3-dihydrobenzo[d]thiazole: A Mild, Efficient, and Highly Active in situ Generated Chemoselective Reducing Agent for the One-Pot Synthesis of 5-Monoalkylbarbiturates in Water](/storage/images/resized/xqixcltwJYe6H8Uco2JbAFfIOzt7UNKH0OcPOPzO_small_thumb.webp)
42.
Runikhina S.A., Tsygankov A.A., Afanasyev O.I., Chusov D.
Mendeleev Communications,
2023
43.

Podyacheva E., Balalaeva A.I., Afanasyev O.I., Runikhina S., Chusova O., Kozlov A., Liao S., Chusov D.
New Journal of Chemistry,
2023
44.
Efimova A.S., Ustimova M.A., Maksimova M.A., Frolova A.Y., Martynov V.I., Deyev S.M., Pakhomov A.A., Fedorov Y.V., Fedorova O.A.
Mendeleev Communications,
2023
45.
Kalugin V.E., Adaeva O.I., Demchuk D.V., Semenov V.V.
Mendeleev Communications,
2023
46.
![A 1,3-dipolar cycloaddition of azomethine ylides to imidazo[4,5-e]thiazolo[2,3-c][1,2,4]triazine oxindolylidene derivatives in the synthesis of novel spirooxindole derivatives](/storage/images/resized/voXLqlsvTwv5p3iMQ8Dhs95nqB4AXOG7Taj7G4ra_small_thumb.webp)
Izmest’ev A.N., Kravchenko A.N., Gazieva G.A.
Chemistry of Heterocyclic Compounds,
2023
47.

Gulyaeva E.S., Osipova E.S., Kovalenko S.A., Filippov O.A., Belkova N., Vendier L., CANAC Y., Shubina E., Valyaev D.A.
Chemical Science,
2024
48.

Smirnov I.V., Biriukov K.O., Shvydkiy N.V., Perekalin D.S., Afanasyev O.I., Chusov D.
Journal of Organic Chemistry,
2024