Keywords
Acetylene
aromatic compounds
methane
polyynes
quantum chemical calculations
thermodynamic analysis
Abstract
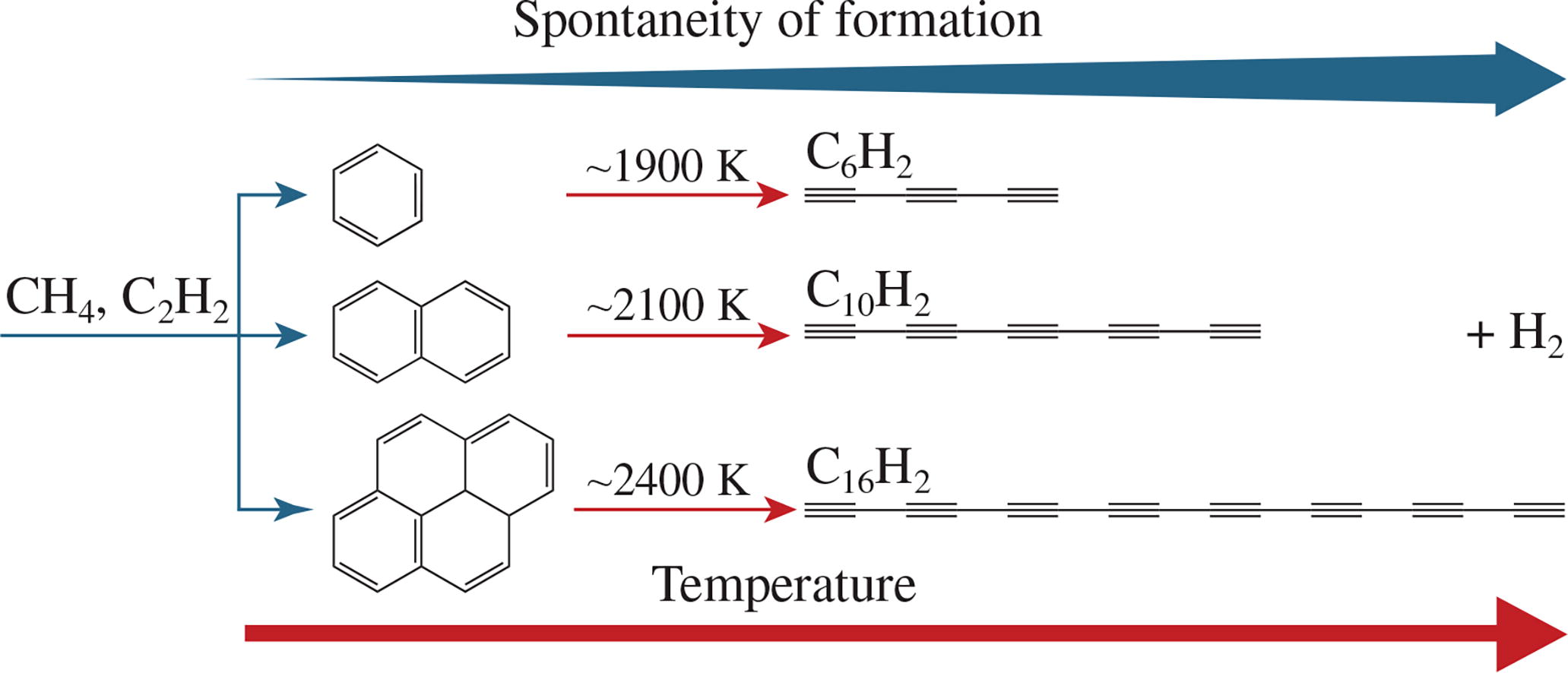
The Gibbs free energies of the formation of several polyynes (C6H2, C10H2 and C16H2) and aromatic species (C6H6, C10H8 and C16H10) from methane and acetylene at temperatures of 1000–2600 K and atmospheric pressure were obtained by quantum chemical calculations using the RI-MP2 method in the ORCA open source software. At lower temperatures, aromatic species form more readily than polyynes, while at temperatures >2200 K the trend reverses and polyyne formation becomes predominant.
References
1.

Feyereisen M., Fitzgerald G., Komornicki A.
Chemical Physics Letters,
1993
2.

Woon D.E., Dunning T.H.
Journal of Chemical Physics,
1994
3.

Neese F., Wennmohs F., Becker U., Riplinger C.
Journal of Chemical Physics,
2020
4.

Wang H.
Proceedings of the Combustion Institute,
2011
5.

Frenklach M., Wang H.
Symposium (International) on Combustion,
1991
6.

Sabbah H., Biennier L., Klippenstein S.J., Sims I.R., Rowe B.R.
Journal of Physical Chemistry Letters,
2010
7.

von Helden G., Hsu M.T., Gotts N., Bowers M.T.
The Journal of Physical Chemistry,
1993
8.

Agafonov G.L., Bilera I.V., Vlasov P.A., Zhil’tsova I.V., Kolbanovskii Y.A., Smirnov V.N., Tereza A.M.
Kinetics and Catalysis,
2016
9.

Haynes B.S., Wagner H.G.
Progress in Energy and Combustion Science,
1981
10.

Guéret C., Daroux M., Billaud F.
Chemical Engineering Science,
1997
11.

Krestinin A.V.
Symposium (International) on Combustion,
1998
12.

Homann K.H., Wagner H.G.
Symposium (International) on Combustion,
1967
13.

MINUTOLO P., GAMBI G., D'ALESSIO A., D'ANNA A.
Combustion Science and Technology,
1994
14.

Krestinin A.V.
Combustion and Flame,
2000
15.

Ranzi E., Dente M., Goldaniga A., Bozzano G., Faravelli T.
Progress in Energy and Combustion Science,
2001
16.

Bonne U., Homann K.H., Wagner H.G.
Symposium (International) on Combustion,
1965
17.

Martin J.W., Salamanca M., Kraft M.
Progress in Energy and Combustion Science,
2022
18.

Siegmann K., Sattler K., Siegmann H.C.
Journal of Electron Spectroscopy and Related Phenomena,
2002
19.

Mironenko R.M., Likholobov V.A., Belskaya O.B.
Russian Chemical Reviews,
2022
20.

Makaryan I.A., Salgansky E.A., Arutyunov V.S., Sedov I.V.
Energies,
2023
21.
Busillo E., Vlasov P., Arutyunov V.
Mendeleev Communications,
2022