Keywords
[1
2
4]triazolo[1
5-a]pyrimidines
acrylonitrile
DFT.
dinitropyridines
nitro synthons
pyrazolo[1
Abstract
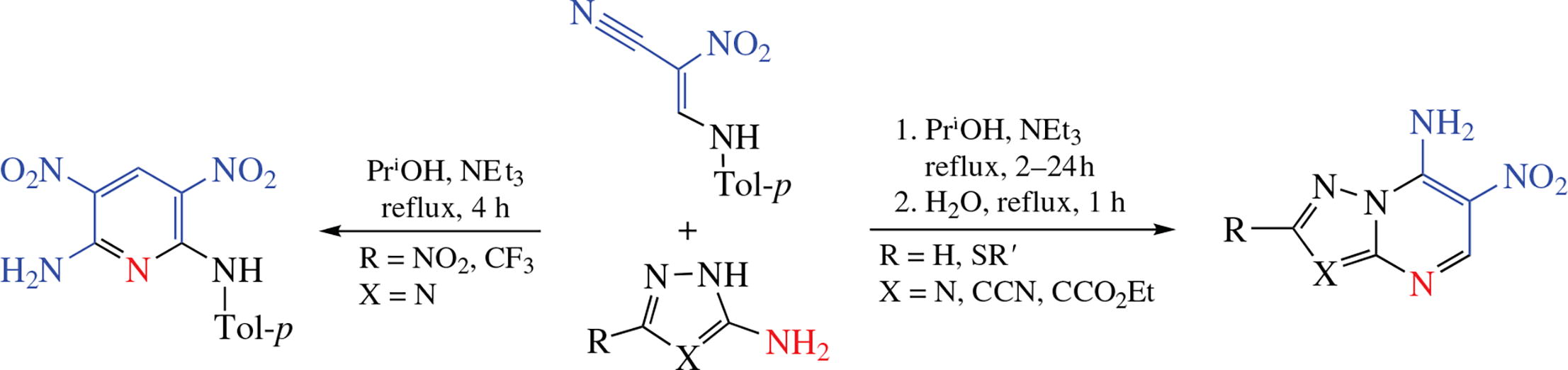
New 7-amino-6-nitro-substituted [1,2,4]triazolo- and pyrazolo[1,5-a]pyrimidines were synthesized by an alternative strategy based on amino azoles and 2-nitro-3-(p-tolylamino)acrylonitrile. Unexpectedly, 3,5-dinitro-N-(p-tolyl)pyridine-2,6-diamine was formed when the starting 5-amino-1,2,4-triazoles contained NO2 or CF3 substituents.
References
.

Grimme S., Ehrlich S., Goerigk L.
Journal of Computational Chemistry,
2011
.

Dolomanov O.V., Bourhis L.J., Gildea R.J., Howard J.A., Puschmann H.
Journal of Applied Crystallography,
2009
.

Neese F., Wennmohs F., Becker U., Riplinger C.
Journal of Chemical Physics,
2020
.

Neese F.
Wiley Interdisciplinary Reviews: Computational Molecular Science,
2011
.

Rusinov V.L., Charushin V.N., Chupakhin O.N.
Russian Chemical Bulletin,
2018
.

Deev S.L., Khalymbadzha I.A., Shestakova T.S., Charushin V.N., Chupakhin O.N.
RSC Advances,
2019
.
![1,2,4-Triazolo[1,5-a]pyrimidines in drug design.](/storage/images/resized/GDnYOu1UpMMfMMRV6Aqle4H0YLLsraeD9IP9qScG_small_thumb.webp)
Oukoloff K., Lucero B., Francisco K.R., Brunden K.R., Ballatore C.
European Journal of Medicinal Chemistry,
2019
.
![Pyrazolo[1,5‐a]pyrimidines. A combined multinuclear magnetic resonance (1H, 13C, 15N, 19F) and DFT approach to their structural assignment](/storage/images/resized/bRyGpdm98BkAUYiK1YFNpl5Z7hPu6Gd87gbIeuG3_small_thumb.webp)
Sanz D., Claramunt R.M., Saini A., Kumar V., Aggarwal R., Singh S.P., Alkorta I., Elguero J.
Magnetic Resonance in Chemistry,
2007
.
![Biological activities of [1,2,4]triazolo[1,5-a]pyrimidines and analogs](/storage/images/resized/voXLqlsvTwv5p3iMQ8Dhs95nqB4AXOG7Taj7G4ra_small_thumb.webp)
Pinheiro S., Pinheiro E.M., Muri E.M., Pessôa J.C., Cadorini M.A., Greco S.J.
Medicinal Chemistry Research,
2020
.
![Synthesis and evaluation of anti-tumor activity of novel triazolo[1,5-a] pyrimidine on cancer cells by induction of cellular apoptosis and inhibition of epithelial-to-mesenchymal transition process](/storage/images/resized/GDnYOu1UpMMfMMRV6Aqle4H0YLLsraeD9IP9qScG_small_thumb.webp)
Safari F., Bayat M., Nasri S., Karami S.
Bioorganic and Medicinal Chemistry Letters,
2020
.
![A New Family of Fused Azolo[1,5-a]pteridines and Azolo[5,1-b]purines](/storage/images/resized/iLiQsFqFaSEx6chlGQ5fbAwF6VYU3WWa08hkss0g_small_thumb.webp)
Gazizov D.A., Gorbunov E.B., Rusinov G.L., Ulomsky E.N., Charushin V.N.
ACS Omega,
2020
.
![2-R-7-methyl[1,2,4]triazolo[2,3-a]pyrimidines: synthesis and structures](/storage/images/resized/voXLqlsvTwv5p3iMQ8Dhs95nqB4AXOG7Taj7G4ra_small_thumb.webp)
Shikhaliev K.S., Krylski D.V., Potapov A.Y., Nefedov S.E., Sidorenko O.E.
Russian Chemical Bulletin,
2008
.
![Synthesis and enzymic activity of 6-carbethoxy- and 6-ethoxy-3,7-disubstituted pyrazolo[1,5-a]pyrimidines and related derivatives as adenosine cyclic 3',5'-phosphate phosphodiesterase inhibitors](/storage/images/resized/iLiQsFqFaSEx6chlGQ5fbAwF6VYU3WWa08hkss0g_small_thumb.webp)
Springer R.H., Scholten M.B., O'Brien D.E., Novinson T., Miller J.P., Robins R.K.
Journal of Medicinal Chemistry,
1982
.

Parry D., Guzi T., Shanahan F., Davis N., Prabhavalkar D., Wiswell D., Seghezzi W., Paruch K., Dwyer M.P., Doll R., Nomeir A., Windsor W., Fischmann T., Wang Y., Oft M., et. al.
Molecular Cancer Therapeutics,
2010
.

Chupakhin O.N., Rusinov V.L., Varaksin M.V., Ulomskiy E.N., Savateev K.V., Butorin I.I., Du W., Sun Z., Charushin V.N.
International Journal of Molecular Sciences,
2022
.
![Differentiation between [1,2,4]triazolo[1,5-a] pyrimidine and [1,2,4]triazolo[4,3-a]- pyrimidine regioisomers by 1H15N HMBC experiments](/storage/images/resized/bRyGpdm98BkAUYiK1YFNpl5Z7hPu6Gd87gbIeuG3_small_thumb.webp)
Salgado A., Varela C., García Collazo A.M., Pevarello P.
Magnetic Resonance in Chemistry,
2010
.
![Methylenaktive Nitroverbindungen, 3. Darstellung von 3‐Amino‐2‐nitroacrylsäure‐Derivaten und Nitro‐4 H ‐pyrido[1,2‐ a ]pyrimidinen aus Nitroessigsäure‐ethylester](/storage/images/resized/bRyGpdm98BkAUYiK1YFNpl5Z7hPu6Gd87gbIeuG3_small_thumb.webp)
.
![5,7-Disubstituted-[1,2,4]triazolo[1,5- a ][1,3,5]triazines as pharmacological tools to explore the antagonist selectivity profiles toward adenosine receptors](/storage/images/resized/GDnYOu1UpMMfMMRV6Aqle4H0YLLsraeD9IP9qScG_small_thumb.webp)
Federico S., Ciancetta A., Porta N., Redenti S., Pastorin G., Cacciari B., Klotz K.N., Moro S., Spalluto G.
European Journal of Medicinal Chemistry,
2016
.
Voinkov E.K., Ulomskiy E.N., Rusinov V.L., Savateev K.V., Fedotov V.V., Gorbunov E.B., Isenov M.L., Eltsov O.S.
Mendeleev Communications,
2016
.

Wendt M.D., Kunzer A., Henry R.F., Cross J., Pagano T.G.
Tetrahedron Letters,
2007
.

Goryaeva M.V., Burgart Y.V., Ezhikova M.A., Kodess M.I., Saloutin V.I.
Beilstein Journal of Organic Chemistry,
2015
.
![A Versatile Method for the Synthesis of 7-Aminoazolo[1,5-a]pyrimidine-6-carbonitriles](/storage/images/resized/oZgeErrVFhuDksyqFURLvYS1wtVSBWczh001igGo_small_thumb.webp)
Urakov G.V., Savateev K.V., Rusinov V.L.
Doklady Chemistry,
2022
.
E. M. Mekky A., M. H. Sanad S.
Mendeleev Communications,
2023
.

Lyapustin Daniil N., Fedotov Victor V., Ulomsky Evgeny N., Rusinov Vladimir L., Chupakhin Oleg N.
Russian Chemical Reviews,
2023
.
![5-Alkylamino-7-aminoazolo[1,5-a]pyrimidine-6-carbonitriles: synthetic strategies and anticancer activity in vitro](/storage/images/resized/voXLqlsvTwv5p3iMQ8Dhs95nqB4AXOG7Taj7G4ra_small_thumb.webp)
Urakov G.V., Savateev K.V., Melekhin V.V., Kotovskaya S.K., Rusinov V.L.
Russian Chemical Bulletin,
2023