Keywords
1
2-(2-hydroxybenzylideneamino)malonates
3-b]pyrroles
3a
4
4-arylidene-2-phenyloxazol-5(4H)-ones.
9b-tetrahydrochromeno[4
domino reactions
liquid carbon dioxide
organocatalysis
squaramides
Abstract
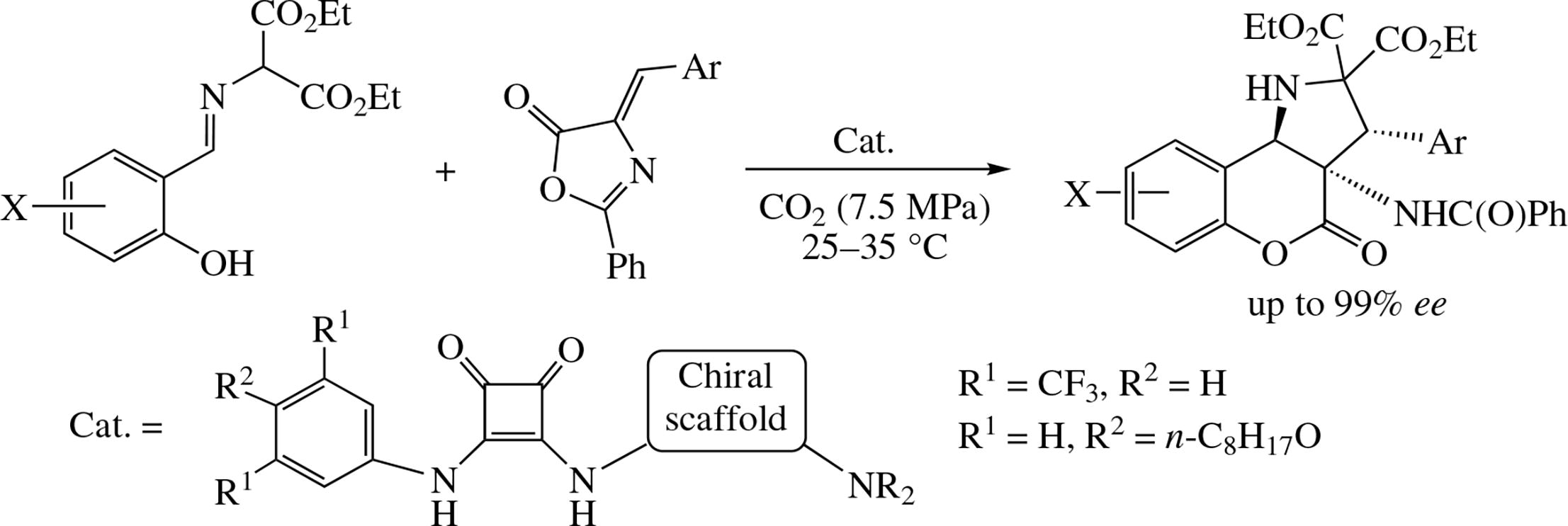
Asymmetric cycloaddition/intramolecular rearrangement domino reaction of 2-(2-hydroxybenzylideneamino)- malonates with 4-arylidene-2-phenyloxazol-5(4H)-ones can be efficiently carried out in sub- or supercritical carbon dioxide to afford (3R,3aS,9bR)-3-aryl-3a-benzamido-4-oxo-1,3a,4,9b-tetrahydrochromeno[4,3-b]pyrrole-2,2(3H)- dicarboxylates in high yields with up to 99% ee. Excellent stereoinduction is provided in this process by the use of bifunctional hybrid organocatalyst consisting of squaramide (thiourea) and chiral tertiary amine units.
References
.

Alekseev E.S., Alentiev A.Y., Belova A.S., Bogdan V.I., Bogdan T.V., Bystrova A.V., Gafarova E.R., Golubeva E.N., Grebenik E.A., Gromov O.I., Davankov V.A., Zlotin S.G., Kiselev M.G., Koklin A.E., Kononevich Y.N., et. al.
Russian Chemical Reviews,
2020
.

Zlotin Sergei G., Egorova Ksenia S., Ananikov Valentine P., Akulov Alexey A., Varaksin Mikhail V., Chupakhin Oleg N., Charushin Valery N., Bryliakov Konstantin P., Averin Alexey D., Beletskaya Irina P., Dolengovski Egor L., Budnikova Yulia H., Sinyashin Oleg G., Gafurov Zufar N., Kantyukov Artyom O., et. al.
Russian Chemical Reviews,
2024
.

Alcalde S., Porcar R., De La Puente M.L., Cumming G.R., Mateos C., García-Losada P., Anta C., Rincón J.A., García-Verdugo E.
Organic Process Research and Development,
2023
.

Dai W., Jiang X., Wu Q., Shi F., Tu S.
Journal of Organic Chemistry,
2015
.

Najera C., Sansano J.
Current Organic Chemistry,
2003
.

Yu J., Chien H., Lin Y., Karanam P., Chen Y., Lin W.
Chemical Communications,
2018
.

Kowalczyk D., Albrecht Ł.
Journal of Organic Chemistry,
2016
.

Filatova E.V., Turova O.V., Nigmatov A.G., Zlotin S.G.
Tetrahedron,
2018
.

Wei L., Chang X., Wang C.
Accounts of Chemical Research,
2020
.

Ren N., Zhang L., Hu Y., Wang X., Deng Z., Chen J., Deng H., Zhang H., Tang X., Cao W.
Journal of Organic Chemistry,
2021
.
![Hydrogen-Bonding Network Promoted [3+2] Cycloaddition: Asymmetric Catalytic Construction of Spiro-pseudoindoxyl Derivatives](/storage/images/resized/bRyGpdm98BkAUYiK1YFNpl5Z7hPu6Gd87gbIeuG3_small_thumb.webp)
Zhang L., Wang Y., Hu X., Xu P.
Chemistry - An Asian Journal,
2016
.

Cao J., Fang R., Liu J., Lu H., Luo Y., Xu P.
Chemistry - A European Journal,
2018
.

Lyubimov S.E., Cherkasova P.V., Chowdhury B.
Russian Chemical Bulletin,
2022
.
![An efficient strategy for the synthesis of polysubstituted chromeno[4,3-b]pyrrolidine derivatives](/storage/images/resized/leiAYcRDGTSl5B1eCnwpSGqmDEUEfDPPoYisFGhT_small_thumb.webp)
Tian L., Xu G., Li Y., Liang Y., Xu P.
Chemical Communications,
2014
.

Yang S., Reddy G.M., Wang T., Yeh Y., Wang M., Lin W.
Chemical Communications,
2017
.

Huang W., Chen Q., Lin N., Long X., Pan W., Xiong Y., Weng J., Lu G.
Organic Chemistry Frontiers,
2017
.

Fauziev R.V., Ivanov R.E., Kuchurov I.V., Zlotin S.G.
Green Chemistry,
2021
.
![Cascade reaction of o-enoyl arylisocyanide and o-hydroxy aromatic aldimine: diastereoselective access to a polycyclic spirobenzoxazine chromeno[4,3-b]pyrrole derivative](/storage/images/resized/leiAYcRDGTSl5B1eCnwpSGqmDEUEfDPPoYisFGhT_small_thumb.webp)
Xiao Y., Peng X., Shen J., Cui L., Lu S., Jia X., Li C., Li J.
Chemical Communications,
2022
.

Kuchurov I.V., Zharkov M.N., Zlotin S.G.
CrystEngComm,
2022
.

Kowalska E., Sieroń L., Albrecht A.
Molecules,
2022
.
![Organocatalytic asymmetric synthesis of pyrrolo[3,2-c]quinolines via a formal [3+2] cycloaddition-lactamization cascade reaction using a bifunctional squaramide catalyst](/storage/images/resized/GDnYOu1UpMMfMMRV6Aqle4H0YLLsraeD9IP9qScG_small_thumb.webp)
Mahto P., Shukla K., Das A., Singh V.K.
Tetrahedron,
2021
.
Budkova A.V., Merkulov V.G., Ivanov R.E., Zharkov M.N., Kuchurov I.V., Zlotin S.G.
Mendeleev Communications,
2023
.
![Organocatalytic Asymmetric Domino [3+2]‐Cycloaddition‐Acyl Transfer Reaction between Azomethine Ylides and α‐Nitro‐α,β‐Unsaturated Ketones](/storage/images/resized/bRyGpdm98BkAUYiK1YFNpl5Z7hPu6Gd87gbIeuG3_small_thumb.webp)
Sahoo S.C., Parida C., Pan S.C.
Chemistry - An Asian Journal,
2023
.
![Alkylidene Meldrum's Acid as Acceptor‐Donor‐Acceptor with Azomethine Ylide for Organocatalytic Asymmetric (3+2) Cycloaddition/Annulation: Synthesis of Chromeno[4,3‐b]pyrrolidine](/storage/images/resized/bRyGpdm98BkAUYiK1YFNpl5Z7hPu6Gd87gbIeuG3_small_thumb.webp)
Liou Y., Chen Y., Hsu C., Huang X., Wang H., Lin W.
Advanced Synthesis and Catalysis,
2023
.
![Synthesis of chromeno[4,3‐b]pyrrolidines from azomethine ylides and vinyl para‐quinone methides via (3 + 2) cycloaddition/oxa‐1,6‐addition](/storage/images/resized/bRyGpdm98BkAUYiK1YFNpl5Z7hPu6Gd87gbIeuG3_small_thumb.webp)
Wu P., Liou Y., Marri G., Chen Y., Wu J., Lin W.
Journal of the Chinese Chemical Society,
2023