Keywords
alkyne
Annulation
isoquinoline
Pyrrole
zwitteriones.
Abstract
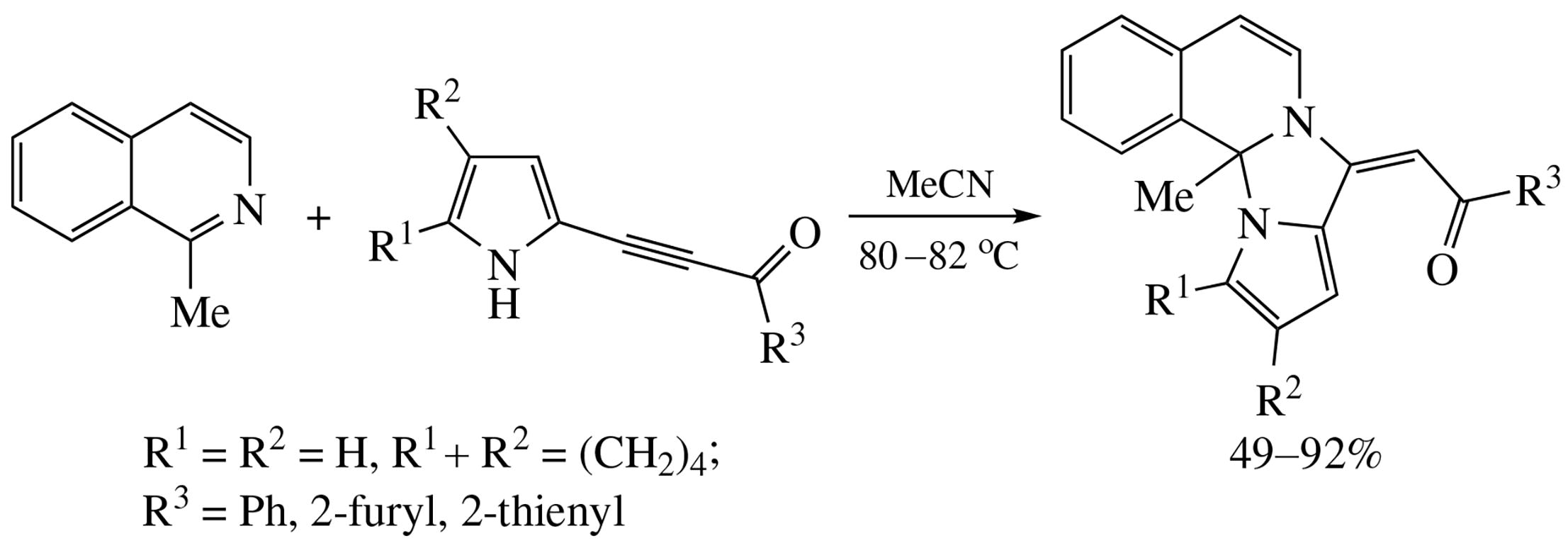
1-Methylisoquinoline undergoes stereoselective annulation with pyrrolylacetylenic ketones (MeCN, 80-82 °C) to provide (E)-acylethenylpyrrolo[1’,2’:3,4]imidazo[2,1-a]isoquinolines in up to 92% yield. In the case of 5-arylpyrrolylacetylenic ketones, instead of the above cyclization, the dimerization of the starting ketones to give dipyrrolopyrazines in 38 and 39% yields occurs.
References
.

Trofimov B.A., Stepanova Z.V., Sobenina L.N., Mikhaleva A.I., Ushakov I.A.
Tetrahedron Letters,
2004
.

Tukhtaev H.B., Ivanov K.L., Bezzubov S.I., Cheshkov D.A., Melnikov M.Y., Budynina E.M.
Organic Letters,
2019
.

Afonin A.V., Ushakov I.A., Pavlov D.V., Ivanov A.V., Mikhaleva A.I.
Magnetic Resonance in Chemistry,
2010
.
![Synthesis, Antibacterial and Antifungal Activity of New 3-Aryl-5H-pyrrolo[1,2-a]imidazole and 5H-Imidazo[1,2-a]azepine Quaternary Salts](/storage/images/resized/MjH1ITP7lMYGxeqUZfkt2BnVLgjkk413jwBV97XX_small_thumb.webp)
Demchenko S., Lesyk R., Yadlovskyi O., Zuegg J., Elliott A.G., Drapak I., Fedchenkova Y., Suvorova Z., Demchenko A.
Molecules,
2021
.
![Rapid Syntheses of Heteroaryl-Substituted Imidazo[1,5-a]indole and Pyrrolo[1,2-c]imidazole via Aerobic C2–H Functionalizations](/storage/images/resized/iLiQsFqFaSEx6chlGQ5fbAwF6VYU3WWa08hkss0g_small_thumb.webp)
Kong W., Chen X., Wang M., Dai H., Yu J.
Organic Letters,
2017
.
![Three Sequential C–N Bond Formations: tert-Butyl Nitrite as a N1 Synthon in a Three Component Reaction Leading to Imidazo[1,2-a]quinolines and Imidazo[2,1-a]isoquinolines](/storage/images/resized/iLiQsFqFaSEx6chlGQ5fbAwF6VYU3WWa08hkss0g_small_thumb.webp)
Sau P., Rakshit A., Modi A., Behera A., Patel B.K.
Journal of Organic Chemistry,
2018
.
![Catalyst-Free Annulation of Acylethynylpyrroles with 1-Pyrrolines: A Straightforward Access to Tetrahydrodipyrrolo[1,2-a:1′,2′-c]imidazoles](/storage/images/resized/iLiQsFqFaSEx6chlGQ5fbAwF6VYU3WWa08hkss0g_small_thumb.webp)
Oparina L.A., Belyaeva K.V., Kolyvanov N.A., Ushakov I.A., Gotsko M.D., Sobenina L.N., Vashchenko A.V., Trofimov B.A.
Journal of Organic Chemistry,
2022
.
![Iodine-Mediated Multicomponent Cascade Cyclization and Sulfenylation/Selenation: Synthesis of Imidazo[2,1-a]isoquinoline Derivatives](/storage/images/resized/iLiQsFqFaSEx6chlGQ5fbAwF6VYU3WWa08hkss0g_small_thumb.webp)
Wang L., Zhou Y., Lei S., Yu X., Huang C., Wu Y., Wu A.
Organic Letters,
2022
.
![Synthesis of imidazo and benzimidazo[2,1-a]isoquinolines by rhodium-catalyzed intramolecular double C–H bond activation](/storage/images/resized/leiAYcRDGTSl5B1eCnwpSGqmDEUEfDPPoYisFGhT_small_thumb.webp)
Reddy V.P., Iwasaki T., Kambe N.
Organic and Biomolecular Chemistry,
2013
.
![Cobalt-catalyzed oxidative [3 + 2] cycloaddition reactions: an efficient synthesis of pyrrolo- and imidazo-[2,1-a]isoquinolines](/storage/images/resized/leiAYcRDGTSl5B1eCnwpSGqmDEUEfDPPoYisFGhT_small_thumb.webp)
Feng C., Su J., Yan Y., Guo F., Wang Z.
Organic and Biomolecular Chemistry,
2013
.
![Diversity-oriented synthesis of imidazo[2,1-a]isoquinolines](/storage/images/resized/leiAYcRDGTSl5B1eCnwpSGqmDEUEfDPPoYisFGhT_small_thumb.webp)
Mai S., Luo Y., Huang X., Shu Z., Li B., Lan Y., Song Q.
Chemical Communications,
2018
.
![Antitumor Activity of 5-Aryl-2,3-dihydroimidazo[2,1-a]isoquinolines](/storage/images/resized/iLiQsFqFaSEx6chlGQ5fbAwF6VYU3WWa08hkss0g_small_thumb.webp)
Houlihan W.J., Munder P.G., Handley D.A., Cheon S.H., Parrino V.A.
Journal of Medicinal Chemistry,
1995
.
![1-Methylimidazole as an Organic Catalyst for [3+3]-Cyclodimerization of Acylethynylpyrroles to Bis(acylmethylidene)dipyrrolo[1,2-a:1′,2′-d]pyrazines](/storage/images/resized/MjH1ITP7lMYGxeqUZfkt2BnVLgjkk413jwBV97XX_small_thumb.webp)
Belyaeva K.V., Nikitina L.P., Gen’ V.S., Tomilin D.N., Sobenina L.N., Afonin A.V., Oparina L.A., Trofimov B.A.
Catalysts,
2022
.
![Superacid-Promoted Cyclodehydration Leading to the Imidazo[2,1-a]isoquinoline Ring System](/storage/images/resized/bRyGpdm98BkAUYiK1YFNpl5Z7hPu6Gd87gbIeuG3_small_thumb.webp)
Kethe A., Naredla R.R., Klumpp D.A.
Helvetica Chimica Acta,
2013
.

Afonin A.V., Ushakov I.A., Vashchenko A.V., Simonenko D.E., Ivanov A.V., Vasil'tsov A.M., Mikhaleva A.I., Trofimov B.A.
Magnetic Resonance in Chemistry,
2009
.
![Transition-Metal-Free Synthesis of Pyrrolo[1,2-a]pyrazines via Intramolecular Cyclization of N-Propargyl(pyrrolyl)enaminones](/storage/images/resized/xqixcltwJYe6H8Uco2JbAFfIOzt7UNKH0OcPOPzO_small_thumb.webp)
Sobenina L., Sagitova E., Ushakov I., Trofimov B.
Synthesis,
2017
.
![Novel (2-amino-4-arylimidazolyl)propanoic acids and pyrrolo[1,2-c]imidazoles via the domino reactions of 2-amino-4-arylimidazoles with carbonyl and methylene active compounds](/storage/images/resized/ex6KJoZujZOZFZh7jGfeHauiftuB3CI7iwJVFRDg_small_thumb.webp)
Lipson V.V., Pavlovska T.L., Svetlichnaya N.V., Poryvai A.A., Gorobets N.Y., Van der Eycken E.V., Konovalova I.S., Shiskina S.V., Borisov A.V., Musatov V.I., Mazepa A.V.
Beilstein Journal of Organic Chemistry,
2019
.
Belyaeva K.V., Nikitina L.P., Gen' V.S., Kuzmin A.V., Afonin A.V., Trofimov B.A.
Mendeleev Communications,
2022
.
![Design, Synthesis and Pregnancy-Terminating Activity of 2-Aryl Imidazo[2, 1 -a]isoquinolines](/storage/images/resized/bRyGpdm98BkAUYiK1YFNpl5Z7hPu6Gd87gbIeuG3_small_thumb.webp)
Shang Z., Hu G., Wu T., Fang Y., Yu Q.
Chinese Journal of Chemistry,
2010
.

Peshkov A.A., Gapanenok D., Puzyk A., Amire N., Novikov A.S., Martynova S.D., Kalinin S., Dar’in D., Peshkov V.A., Krasavin M.
Journal of Organic Chemistry,
2023
.

Trofimov B., Oparina L., Belyaeva K., Kolyvanov N., Ushakov I., Tomilin D., Sobenina L., Kuzmin A.
New Journal of Chemistry,
2024
.

Trofimov B., Belyaeva K., Nikitina L., Oparina L., Saliy V., Tomilin D., Kuzmin A., Afonin A.
New Journal of Chemistry,
2024