Keywords
bulk perovskite
kinetics
LaCoO3
microwave activation
N2O decomposition
oxygen vacancies.
supported perovskite
Abstract
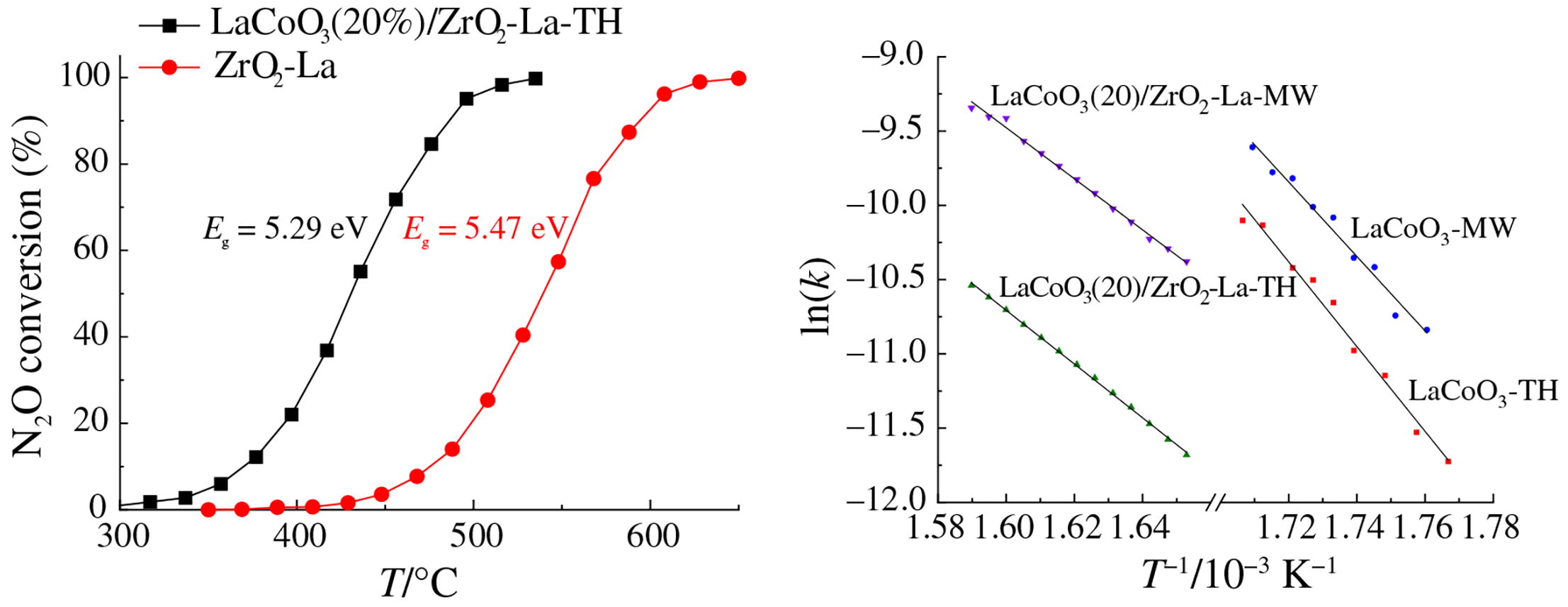
For the first time, kinetic data on the decomposition of N2O over mixed oxide LaCoO3 with a perovskite structure have been obtained. Bulk LaCoO3 synthesized using microwave activation exhibited an increased intrinsic reaction rate with a 30 kJ mol-1 lower activation energy. Perovskite samples supported on ZrO2-La demonstrated lower intrinsic reaction rates due to the lower content of the LaCoO3 phase, but the activation energies were also lower by 50-80 kJ mol-1.
References
.

Tompsett G.A., Conner W.C., Yngvesson K.S.
ChemPhysChem,
2006
.

Granger P., Parvulescu V.I.
Chemical Reviews,
2011
.

Wu Y., Ni X., Beaurain A., Dujardin C., Granger P.
Applied Catalysis B: Environmental,
2012
.

Kostyukhin E.M., Kustov A.L., Evdokimenko N.V., Bazlov A.I., Kustov L.M.
Journal of the American Ceramic Society,
2020
.

Kustov L.M., Dunaev S.F., Kustov A.L.
Molecules,
2022
.

Russo N., Mescia D., Fino D., Saracco G., Specchia V.
Industrial & Engineering Chemistry Research,
2007
.

Huang C., Zhu Y., Wang X., Liu X., Wang J., Zhang T.
Journal of Catalysis,
2017
.

Wu Y., Dujardin C., Lancelot C., Dacquin J.P., Parvulescu V.I., Cabié M., Henry C.R., Neisius T., Granger P.
Journal of Catalysis,
2015
.
Jung W.Y., Hong S.
Journal of Industrial and Engineering Chemistry,
2013
.

Horikoshi S., Osawa A., Sakamoto S., Serpone N.
Chemical Engineering and Processing: Process Intensification,
2013
.

Chagas C.A., Toniolo F.S., Magalhães R.N., Schmal M.
International Journal of Hydrogen Energy,
2012
.

He X., Meng M., He J., Zou Z., Li X., Li Z., Jiang Z.
Catalysis Communications,
2010
.

Villoria J.A., Alvarez-Galvan M.C., Navarro R.M., Briceño Y., Gordillo Alvarez F., Rosa F., Fierro J.L.
Catalysis Today,
2008
.

Lin F., Andana T., Wu Y., Szanyi J., Wang Y., Gao F.
Journal of Catalysis,
2021
.

Pérez-Ramı́rez J., Kapteijn F., Schöffel K., Moulijn J.A.
Applied Catalysis B: Environmental,
2003
.

Campa M.C., Doyle A.M., Fierro G., Pietrogiacomi D.
Catalysis Today,
2022
.

Bozorgi B., Karimi-Sabet J., Khadiv-Parsi P.
Environmental Technology and Innovation,
2022
.

Kang B., Li M., Di Z., Guo X., Wei Y., Jia J., Zhang R.
Catalysis Today,
2022
.

Strekalova A.A., Shesterkina A.A., Kustov A.L., Kustov L.M.
International Journal of Molecular Sciences,
2023
.

Kustov L.M., Kostyukhin E.M., Korneeva E.Y., Kustov A.L.
Russian Chemical Bulletin,
2023
.

Campa M.C., Pietrogiacomi D., Catracchia C., Morpurgo S., Olszowka J., Mlekodaj K., Lemishka M., Dedecek J., Kornas A., Tabor E.
Applied Catalysis B: Environmental,
2024