Keywords
adsorption properties
cucurbit[6]uril
differential capacitance
electrode potential
MgSO4 solutions
stability constant
supramolecular complexes
Abstract
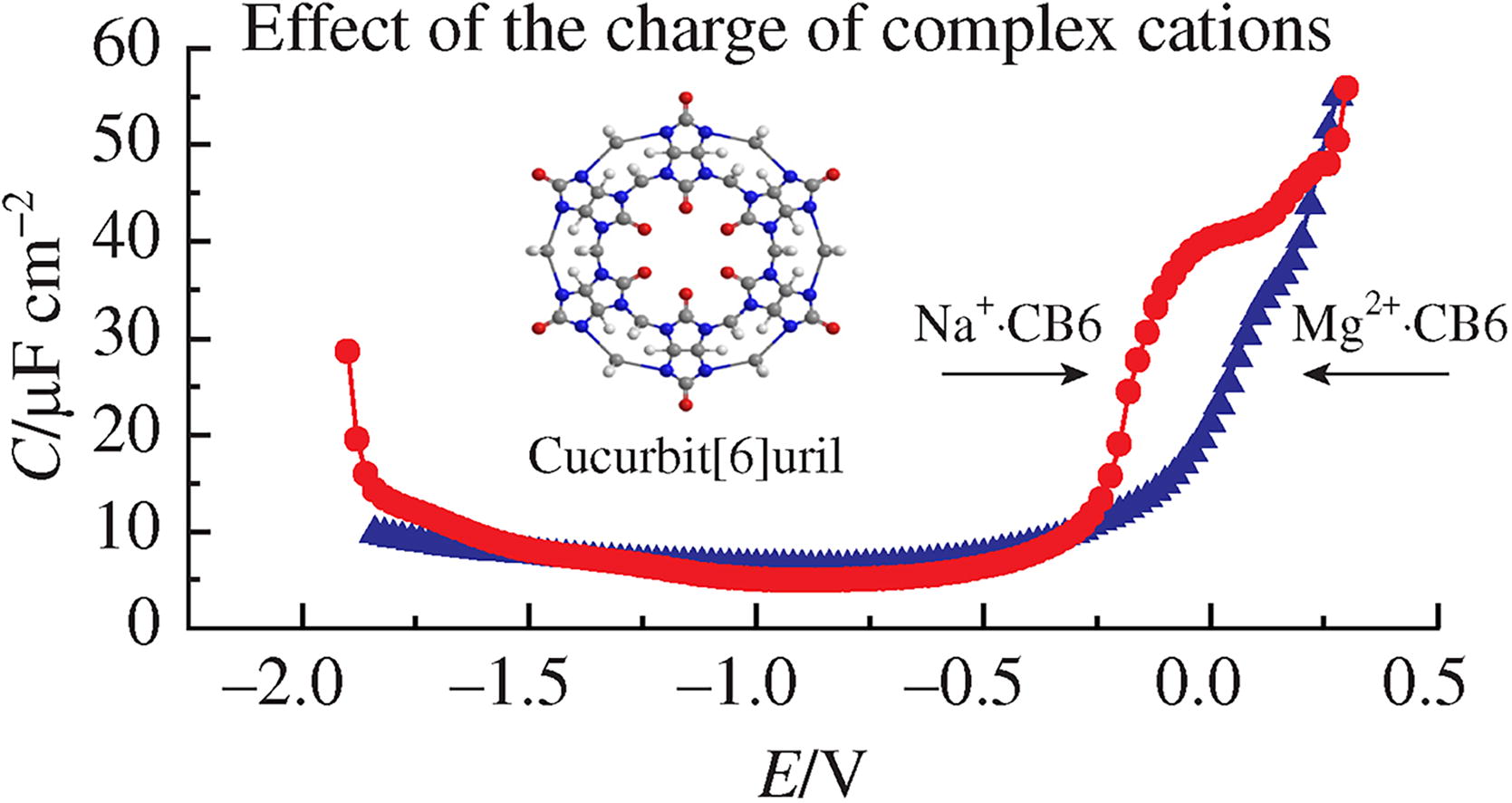
The behavior of cucurbit[6]uril at the electrode/solution interface in the presence of magnesium sulfate was investigated and quantified. It has been established that at the highest positive potential available for the adsorption of organic compounds, a structure is formed that consists entirely of neutral cavitand molecules.
References
1.

Freeman W.A., Mock W.L., Shih N.Y.
Journal of the American Chemical Society,
1981
2.

Masson E., Ling X., Joseph R., Kyeremeh-Mensah L., Lu X.
RSC Advances,
2012
3.
![The formation of alkali and alkaline earth cation complexes with cucurbit[6]uril in aqueous solution: a critical survey of old and new results](/storage/images/resized/voXLqlsvTwv5p3iMQ8Dhs95nqB4AXOG7Taj7G4ra_small_thumb.webp)
Buschmann H., Cleve E., Mutihac L., Schollmeyer E.
Journal of Inclusion Phenomena and Macrocyclic Chemistry,
2009
4.

Assaf K.I., Nau W.M.
Chemical Society Reviews,
2015
5.

Kim K., Selvapalam N., Ko Y.H., Park K.M., Kim D., Kim J.
Chemical Society Reviews,
2007
6.
![Macrocyclic cavitands cucurbit[n]urils: prospects for application in biochemistry, medicine and nanotechnology](/storage/images/resized/9Mus3KG1Tkd7Bwaurt8H3RwWh0CxRlGoO6ng9UK1_small_thumb.webp)
Gerasko O.A., Kovalenko E.A., Fedin V.P.
Russian Chemical Reviews,
2016
7.

Ashwin B.C., Shanmugavelan P., Muthu Mareeswaran P.
Journal of Inclusion Phenomena and Macrocyclic Chemistry,
2020
8.
Stenina E.V., Sviridova L.N., Ivanov D.A.
Mendeleev Communications,
2016
9.
![Determination of complex stabilities with nearly insoluble host molecules: cucurbit[5]uril, decamethylcucurbit[5]uril and cucurbit[6]uril as ligands for the complexation of some multicharged cations in aqueous solution](/storage/images/resized/GDnYOu1UpMMfMMRV6Aqle4H0YLLsraeD9IP9qScG_small_thumb.webp)
Buschmann H.-., Cleve E., Jansen K., Schollmeyer E.
Analytica Chimica Acta,
2001
10.
Stenina E.V., Sviridova L.N.
Mendeleev Communications,
2023
11.

Stenina E.V., Sviridova L.N.
Russian Journal of Electrochemistry,
2022
12.

Stenina E.V., Sviridova L.N.
Inorganica Chimica Acta,
2023