Keywords
azo compounds
azo–hydrazone tautomerism.
benzoquinones
dioxolene ligands
hydrazones
p-iminoquinone
photoresponsive ligands
Abstract
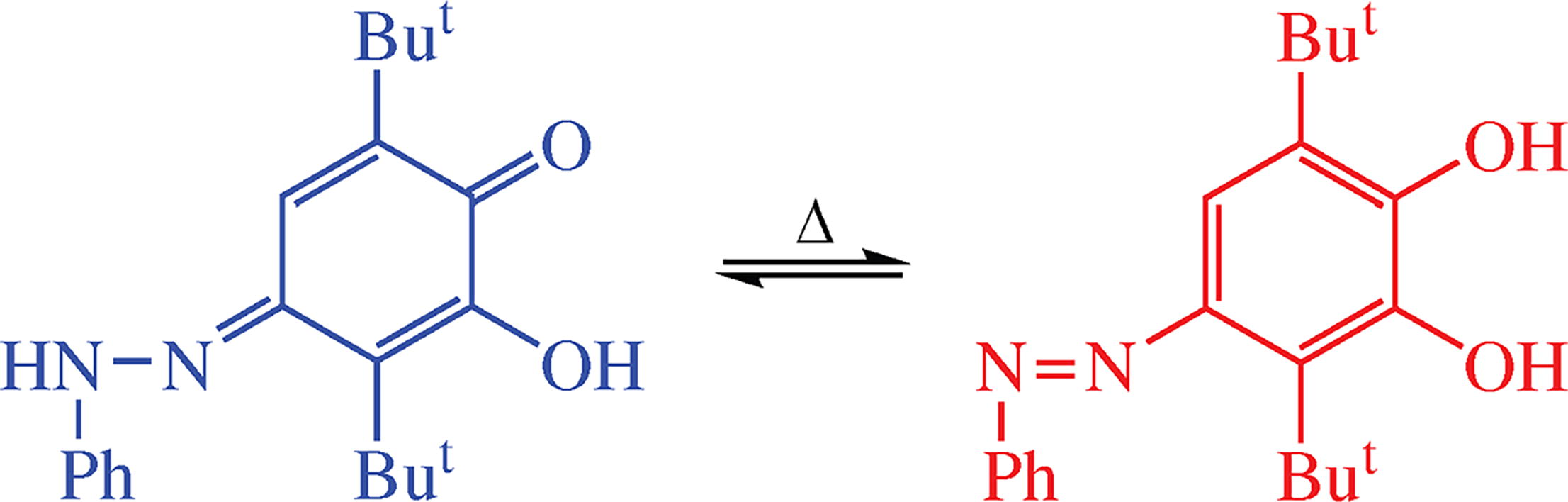
Reaction of 3-hydroxy-2,5-di-tert-butyl-1,4-benzoquinone with phenylhydrazine hydrochloride mainly leads to the corresponding hydrazone at the position 1 existing in tautomeric equilibrium with 3,6-di-tert-butyl-4-(phenyl- diazenyl)catechol. Thermodynamic parameters of the tautomerization were evaluated by NMR spectroscopy. The hydrazone structure was confirmed by XRD study.
References
.

Ryazantsev M.N., Strashkov D.M., Nikolaev D.M., Shtyrov A.A., Panov M.S.
Russian Chemical Reviews,
2021
.

Natali M., Giordani S.
Chemical Society Reviews,
2012
.

Beharry A.A., Woolley G.A.
Chemical Society Reviews,
2011
.

Tezgerevska T., Alley K.G., Boskovic C.
Coordination Chemistry Reviews,
2014
.

Drath O., Boskovic C.
Coordination Chemistry Reviews,
2018
.

Venkataramani S., Jana U., Dommaschk M., Sönnichsen F.D., Tuczek F., Herges R.
Science,
2011
.

Thies S., Sell H., Bornholdt C., Schütt C., Köhler F., Tuczek F., Herges R.
Chemistry - A European Journal,
2012
.

Zolotukhin A.A., Bubnov M.P., Cherkasov V.K., Abakumov G.A.
Russian Journal of Coordination Chemistry/Koordinatsionnaya Khimiya,
2018
.

Hasegawa Y., Kume S., Nishihara H.
Dalton Transactions,
2009
.

Khusniyarov M.M.
Chemistry - A European Journal,
2016
.

Zatsepin T.S., Abrosimova L.A., Monakhova M.V., Le Thi Hien, Pingoud A., Kubareva E.A., Oretskaya T.S.
Russian Chemical Reviews,
2013
.

Crespi S., Simeth N.A., König B.
Nature Reviews Chemistry,
2019
.

Harding D.J., Harding P., Phonsri W.
Coordination Chemistry Reviews,
2016
.

Bléger D., Hecht S.
Angewandte Chemie - International Edition,
2015
.

Haghbeen K., Tan E.W.
Journal of Organic Chemistry,
1998
.

Xie K., Ruan Z., Lyu B., Chen X., Zhang X., Huang G., Chen Y., Ni Z., Tong M.
Angewandte Chemie - International Edition,
2021
.

Kocherova T.N., Druzhkov N.O., Martyanov K.A., Shavyrin A.S., Arsenyev M.V., Kulikova T.I., Baranov E.V., Kuropatov V.A., Cherkasov V.K.
Russian Chemical Bulletin,
2020
.

Novikova I.A., Vol'eva V.B., Komissarova N.L., Belostotskaya I.S., Ershov V.V.
Russian Chemical Bulletin,
1981
.

Chegerev M., Demidov O., Vasilyev P., Efimov N., Kubrin S., Starikov A., Vlasenko V., Piskunov A., Shapovalova S., Guda A., Rusalev Y., Soldatov A.
Dalton Transactions,
2022
.

Smith L.I., Irwin W.B.
Journal of the American Chemical Society,
1941
.

Saeva F.
Journal of Organic Chemistry,
1971
.

Jerca F.A., Jerca V.V., Hoogenboom R.
Nature Reviews Chemistry,
2021
.

Lin H., Wang Y., Shi X., Yang H., Xu L.
Chemical Society Reviews,
2023
.

Himmel H.
Inorganica Chimica Acta,
2018
.

Ball P., Nicholls C.H.
Dyes and Pigments,
1982