Keywords
acetylenic ketones
aldehydes
alkynones
C–H active compounds
triphenylphosphine.
Abstract
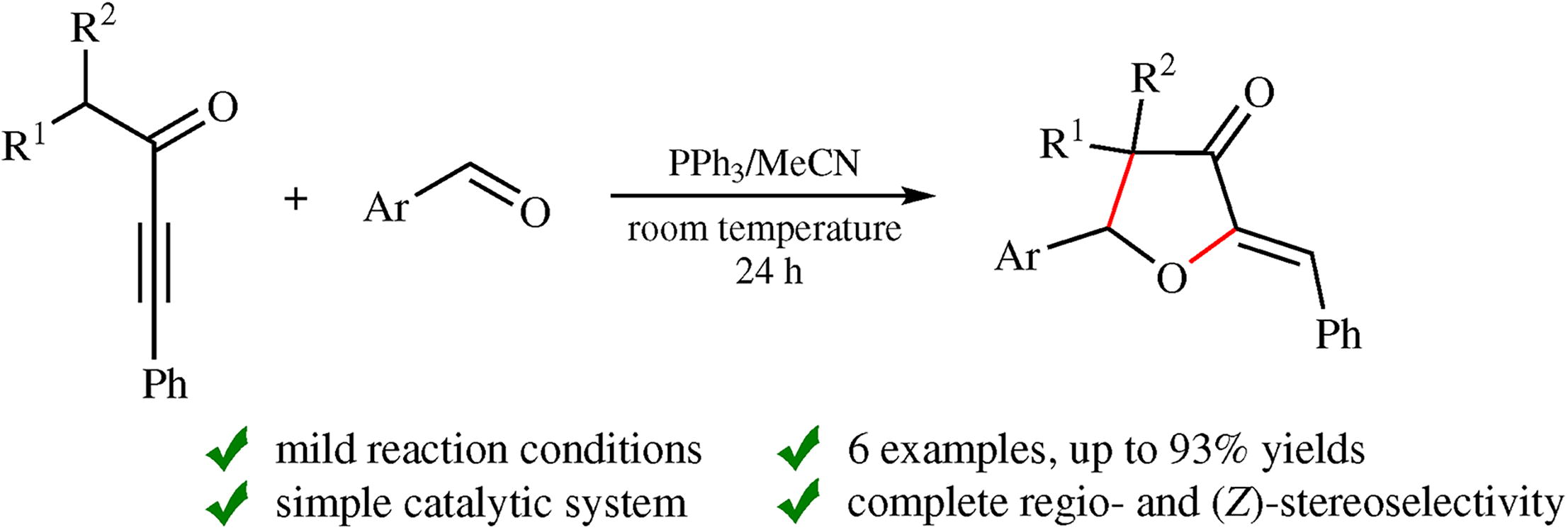
1-Alkyl-3-phenylprop-2-yn-1-ones smoothly react with aromatic aldehydes in the presence of triphenylphosphine (acetonitrile, room temperature, 24 h) to regio- and stereo- selectively afford (Z)-2-benzylideneoxacyclopentan-3-ones in yields up to 93%. The proposed mechanism for the transformation involves 1,3-H proton shift from the alkyl group at the intermediate β-phosphoniovinylide species.
References
.

Nájera C., Sydnes L.K., Yus M.
Chemical Reviews,
2019
.
![Phosphine-Catalyzed [3 + 3]-Domino Cycloaddition of Ynones and Azomethine Imines To Construct Functionalized Hydropyridazine Derivatives.](/storage/images/resized/iLiQsFqFaSEx6chlGQ5fbAwF6VYU3WWa08hkss0g_small_thumb.webp)
Liang L., Huang Y.
Organic Letters,
2016
.

Li Y., Yu J., Bi Y., Yan G., Huang D.
Advanced Synthesis and Catalysis,
2019
.
![Phosphine-Catalyzed α-Umpolung–Aldol Reaction for the Synthesis of Benzo[b]azapin-3-ones](/storage/images/resized/iLiQsFqFaSEx6chlGQ5fbAwF6VYU3WWa08hkss0g_small_thumb.webp)
Zhang K., Cai L., Hong S., Kwon O.
Organic Letters,
2019
.

Worch J.C., Stubbs C.J., Price M.J., Dove A.P.
Chemical Reviews,
2021
.
![Phosphine-Catalyzed Cascade Reaction of Unsaturated Pyrazolones with Alkyne Derivatives: Efficient Synthesis of Pyrano[2,3-c]pyrazoles and Spiro-cyclopentanone-pyrazolones](/storage/images/resized/bRyGpdm98BkAUYiK1YFNpl5Z7hPu6Gd87gbIeuG3_small_thumb.webp)
Li J., Du D.
Advanced Synthesis and Catalysis,
2015
.
![Intramolecular hydrogen-bonding-assisted phosphine-catalysed [3 + 2] cyclisation of ynones with o-hydroxy/amino benzaldehydes](/storage/images/resized/leiAYcRDGTSl5B1eCnwpSGqmDEUEfDPPoYisFGhT_small_thumb.webp)
Deng Z., Xie Z., Zheng Y., Xiao J., Wang R., Xiang H., Yang H.
Organic and Biomolecular Chemistry,
2019
.
![Regio- and stereoselective base-catalyzed assembly of 6-methylene-5-oxaspiro[2.4]heptanones from alkynyl cyclopropyl ketones](/storage/images/resized/leiAYcRDGTSl5B1eCnwpSGqmDEUEfDPPoYisFGhT_small_thumb.webp)
Samultceva S.O., Dvorko M.Y., Shabalin D.A., Ushakov I.A., Vashchenko A.V., Schmidt E.Y., Trofimov B.A.
Organic and Biomolecular Chemistry,
2022
.

Dutta L., Chattopadhyay A., Yadav N., Ramasastry S.S.
Organic and Biomolecular Chemistry,
2023
.

Dvorko M.Y., Shabalin D.A., Ushakov I.A., Schmidt E.Y., Trofimov B.A.
European Journal of Organic Chemistry,
2023