Keywords
nitromethane
nucleophilic substitution
organofluorine compounds.
polyfluoroaromatic compounds
sulfanes
sulfones
Abstract
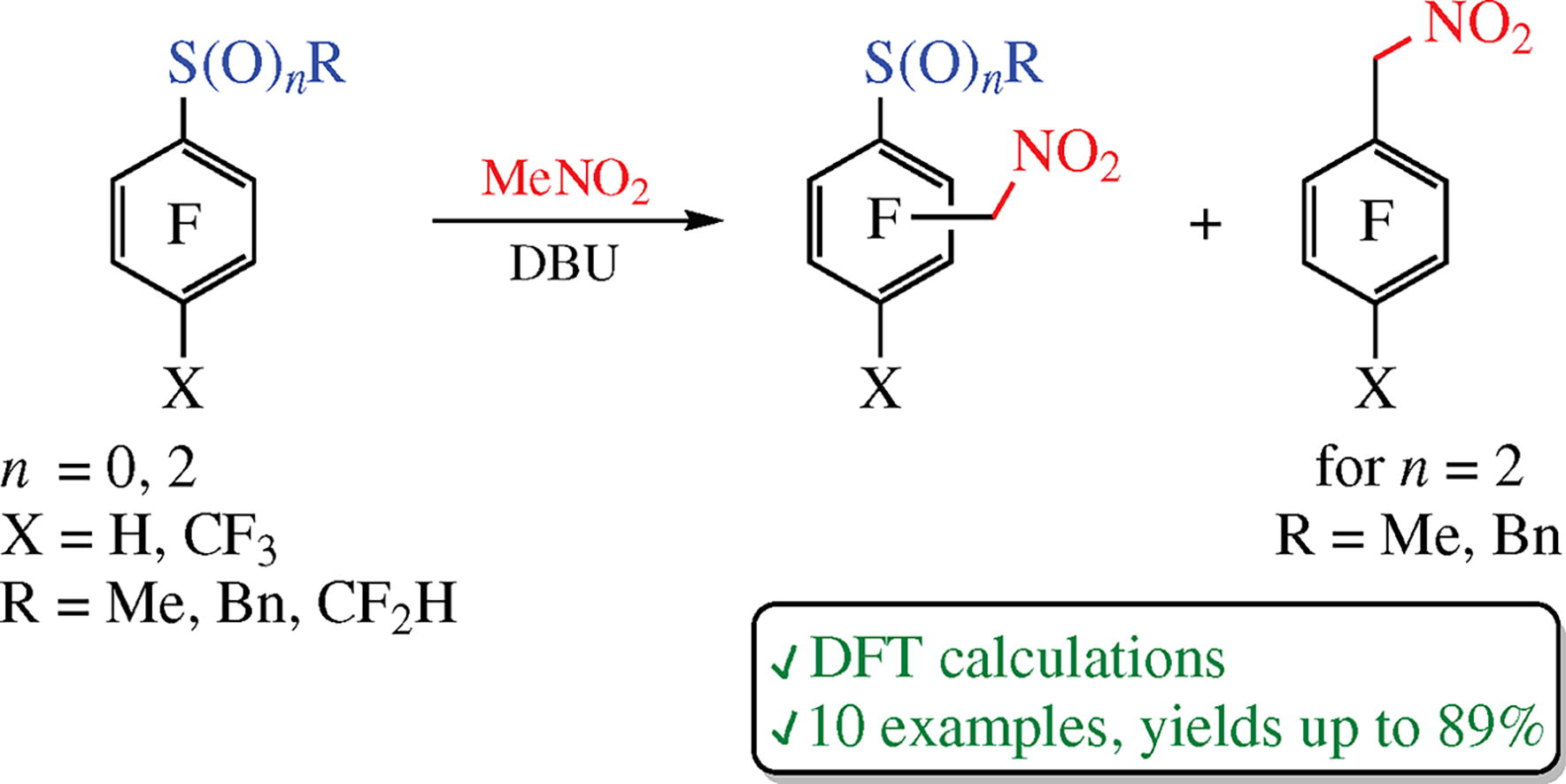
1-Alkylsulfanyl-4-X-2,3,5,6-tetrafluorobenzenes (X = CF3, H; alkyl = Me, Bn, CHF2) upon reactions with nitromethane in the presence of DBU undergo replacement of 2 or 3-positioned fluorine atoms by a nitromethyl group to afford the corresponding (nitromethyl)trifluoroarenes. The calculations of the transition states using the DFT method correctly predict the ratios of reaction products. In the cases of relative sulfones, only 2-positioned fluorine atom undergoes substitution; however, the sulfonyl group can also be displaced by a nitromethyl moiety.
References
.

Politanskaya L.V., Selivanova G.A., Panteleeva E.V., Tretyakov E.V., Platonov V.E., Nikul’shin P.V., Vinogradov A.S., Zonov Y.V., Karpov V.M., Mezhenkova T.V., Vasilyev A.V., Koldobskii A.B., Shilova O.S., Morozova S.M., Burgart Y.V., et. al.
Russian Chemical Reviews,
2019
.

Blockhuys F., Gritsan N.P., Makarov A.Y., Tersago K., Zibarev A.V.
European Journal of Inorganic Chemistry,
2008
.
Buravlev A.A., Makarov A.Y., Rakitin O.A., Zibarev A.V.
Mendeleev Communications,
2023
.

Ballini R., Bosica G., Fiorini D., Palmieri A., Petrini M.
Chemical Reviews,
2005
.

Ballini R., Petrini M.
Advanced Synthesis and Catalysis,
2015
.

Swallow S.
Progress in Medicinal Chemistry,
2015
.

Hansch C., Leo A., Taft R.W.
Chemical Reviews,
1991
.
.

.

Ballari M.S., Herrera Cano N., Wunderlin D.A., Feresin G.E., Santiago A.N.
RSC Advances,
2019
.

Liljenberg M., Brinck T., Herschend B., Rein T., Tomasi S., Svensson M.
Journal of Organic Chemistry,
2012
.
.

Yoon S.C., Kim K.
Journal of Organic Chemistry,
1996
.

Gandler J.R., Saunders O.L., Barbosa R.
Journal of Organic Chemistry,
1997
.

Grinblat J., Ben-Zion M., Hoz S.
Journal of the American Chemical Society,
2001
.

Ando K., Shimazu Y., Seki N., Yamataka H.
Journal of Organic Chemistry,
2011
.

Dianati V., Navals P., Couture F., Desjardins R., Dame A., Kwiatkowska A., Day R., Dory Y.L.
Journal of Medicinal Chemistry,
2018
.

Aminocatalytic Enantioselective 1, 6-Addition of (Nitromethyl)benzenes to α, β, γ, δ-Cyclic Dienones
Feng K., Shen Q., Zheng Y., Xia A., Zhou Z., Tang C., Zhong A., Xu D., Du X.
European Journal of Organic Chemistry,
2019
.

Silyanova E.A., Samet A.V., Salamandra L.K., Khrustalev V.N., Semenov V.V.
European Journal of Organic Chemistry,
2020
.

Marcé P., Lynch J., Blacker A.J., Williams J.M.
Chemical Communications,
2016
.

Koshcheev B.V., Maksimov A.M.
Russian Journal of Organic Chemistry,
2022
.

Takata T., Ishibashi K., Ando W.
Tetrahedron Letters,
1985
.

Seebach D., Lehr F.
Helvetica Chimica Acta,
1979
.

Bonesi S.M., Torriani R., Mella M., Albini A.
European Journal of Organic Chemistry,
1999
.

Cinchonidinium acetate as a convenient catalyst for the asymmetric synthesis of cis-stilbenediamines
Walvoord R.R., Kozlowski M.C.
Tetrahedron Letters,
2015
.

Das P., Tokunaga E., Shibata N.
Tetrahedron Letters,
2017
.

Chernysheva N.B., Maksimenko A.S., Andreyanov F.A., Kislyi V.P., Strelenko Y.A., Khrustalev V.N., Semenova M.N., Semenov V.V.
Tetrahedron,
2017
.

Hassan W., Narayanaperumal S., Gul K., Braga A.L., Rodrigues O.D., da Rocha J.B.
Arabian Journal of Chemistry,
2019
.

Barnych B., Singh N., Negrel S., Zhang Y., Magis D., Roux C., Hua X., Ding Z., Morisseau C., Tantillo D.J., Siegel J.B., Hammock B.D.
European Journal of Medicinal Chemistry,
2020
.

Pažitný A., Solčán T., Végh D.
Journal of Fluorine Chemistry,
2009
.

Zhang Z., Yu A., Zhou W.
Bioorganic and Medicinal Chemistry,
2007
.

Zhao L., Yin W., Sun Y., Sun N., Tian L., Zheng Y., Zhang C., Zhao S., Su X., Zhao D., Cheng M.
European Journal of Medicinal Chemistry,
2021
.

Yin W., Cui H., Jiang H., Zhang Y., Liu L., Wu T., Sun Y., Zhao L., Su X., Zhao D., Cheng M.
European Journal of Medicinal Chemistry,
2022
.
Nikul'shin P.V., Maksimov A.M., Gatilov Y.V., Kovtonyuk V.N., Bredikhin R.A.
Mendeleev Communications,
2023
.

Yang Y., Stanbury D.M.
Inorganic Chemistry,
2024