Keywords
cyclopentadienone
D-glucose
fragrance
hedione
Johnson–Claisen rearrangement.
methyl dihydrojasmonate
Abstract
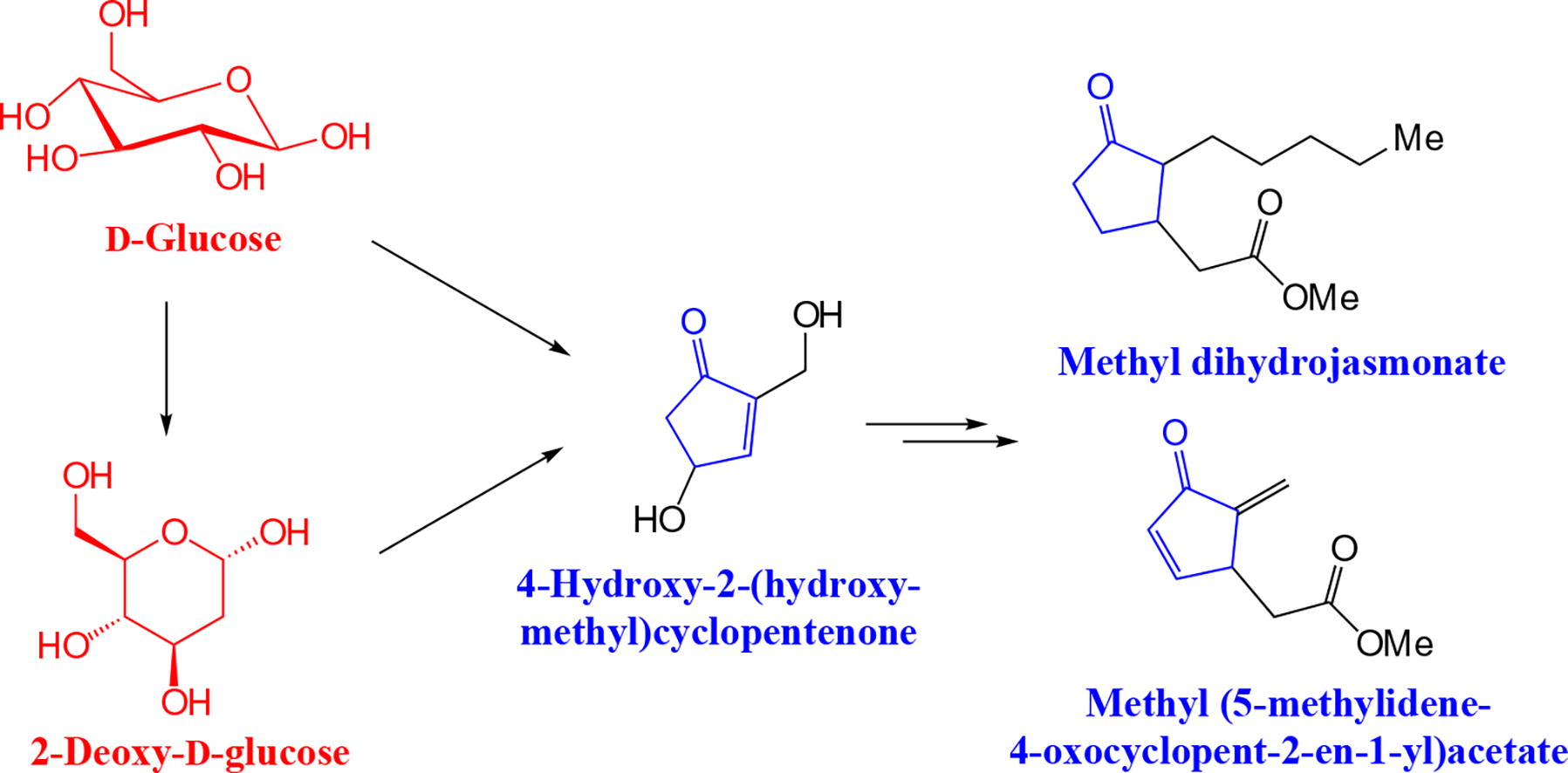
Methyl dihydrojasmonate and methyl (5-methylidene-4-oxocyclopent-2-en-1-yl)acetate were synthesized from 4-hydroxy-2-(hydroxymethyl)cyclopentenone, which, in turn, was obtained from D-glucose. The conditions for the key step, the Johnson-Claisen rearrangement, were optimized to get the maximum yield of the intermediate methyl 2-[2-methylene-3-oxo-5-(triisopropylsilyloxy)-cyclopentyl]acetate.
References
.

Straus D.S., Glass C.K.
Medicinal Research Reviews,
2001
.

Perrard T., Plaquevent J., Desmurs J., Hébrault D.
Organic Letters,
2000
.

Birman V.B., Danishefsky S.J.
Journal of the American Chemical Society,
2002
.

Jágerová D., Šmahel M., Poryvai A., Macháček J., Novotná V., Kohout M.
Crystals,
2021
.

Pützer A., Brüne M., Hatt H., Wolf O.T.
Frontiers in Behavioral Neuroscience,
2020
.

Saeed S., Zahoor A.F., Ahmad S., Akhtar R., Sikandar S.
Synthetic Communications,
2021
.

Pützer A., Wolf O.T.
Stress,
2021
.

Vostrikov N.S., Makaev Z.R., Zagitov V.V., Lakhvich F.A., Pashkovsky F.S., Miftakhov M.S.
Russian Chemical Bulletin,
2020
.

Kuhn C., Roulland E., Madelmont J., Monneret C., Florent J.
Organic and Biomolecular Chemistry,
2004
.

Huang Y., Fu Y.
Green Chemistry,
2013
.

Tsuji J., Kasuga K., Takahashi T.
Bulletin of the Chemical Society of Japan,
1979
.

Koseki Y., Watanabe T., Kamishima T., Kwon E., Kasai H.
Bulletin of the Chemical Society of Japan,
2019
.

Kamishima T., Nonaka T., Watanabe T., Koseki Y., Kasai H.
Bulletin of the Chemical Society of Japan,
2018
.

Kamishima T., Suzuki M., Narita K., Koseki Y., Nonaka T., Nakatsuji H., Hattori H., Kasai H.
Scientific Reports,
2022
.

Morales-delaRosa S., Campos-Martin J.M., Fierro J.L.
Cellulose,
2014
.

Li J., Chen F., Renata H.
Journal of the American Chemical Society,
2022
.

Tsuji J., Kobayashi Y., Kataoka H., Takahashi T.
Tetrahedron Letters,
1980
.

Al’mukhametov A.Z., Aralbaeva G.V., Gimazetdinov A.M.
Russian Journal of Organic Chemistry,
2022
.
Gimazetdinov A.M., Khalfitdinova L.A., Miftakhov M.S.
Mendeleev Communications,
2013
.

Chapuis C.
Helvetica Chimica Acta,
2012
.

Chapuis C., Büchi G., Wüest H.
Helvetica Chimica Acta,
2005
.

Krause N., Ebert S.
European Journal of Organic Chemistry,
2001
.

Giersch W., Farris I.
Helvetica Chimica Acta,
2004
.

Api A.M., Belsito D., Bhatia S., Bruze M., Calow P., Dagli M.L., Dekant W., Fryer A.D., Kromidas L., La Cava S., Lalko J.F., LapczynskI A., Liebler D.C., Miyachi Y., Politano V.T., et. al.
Food and Chemical Toxicology,
2016
.

Ho T., Liu S.
Synthetic Communications,
1982
.

Burstein S.H.
Prostaglandins and Other Lipid Mediators,
2020
.

Scognamiglio J., Jones L., Letizia C.S., Api A.M.
Food and Chemical Toxicology,
2012
.

Szentner K., Waśkiewicz A., Kaźmierczak S., Wojciechowicz T., Goliński P., Lewandowska E., Wasielewski O.
Carbohydrate Research,
2019
.

Gutierrez L.L., Marques C.V., Scomazzon S.P., Schroeder H.T., Fernandes J.R., da Silva Rossato J., Homem de Bittencourt P.I.
Biochimie,
2021
.

Kleoff M., Kiler P., Heretsch P.
Beilstein Journal of Organic Chemistry,
2022
.
Chapuis C., Skuy D., Richard C.
Chimia,
2019
.

Saied M., Gatri R., Al-Ayed A.S., Arfaoui Y., El Gaied M.M.
Letters in Organic Chemistry,
2017
.
Choice Reviews Online,
2012
.

Sharley J.S., Gambacorta G., Pérez A.M., Ferri E.E., Miranda A.F., Quesada J.S., Baxendale I.R.
Data in Brief,
2023
.

Duan Y., Cheng Y., Hu Z., Wang C., Sui D., Yang Y., Lu T.
Molecules,
2023
.

Gimazetdinov A.M., Zagitov V.V., Makaev Z.R., Vostrikov N.S., Miftakhov M.S.
Russian Chemical Bulletin,
2023