Keywords
arylpyridine subunit
crown ring.
dithiaazacrown ethers
Hantzsch synthesis
Hep-G2
Abstract
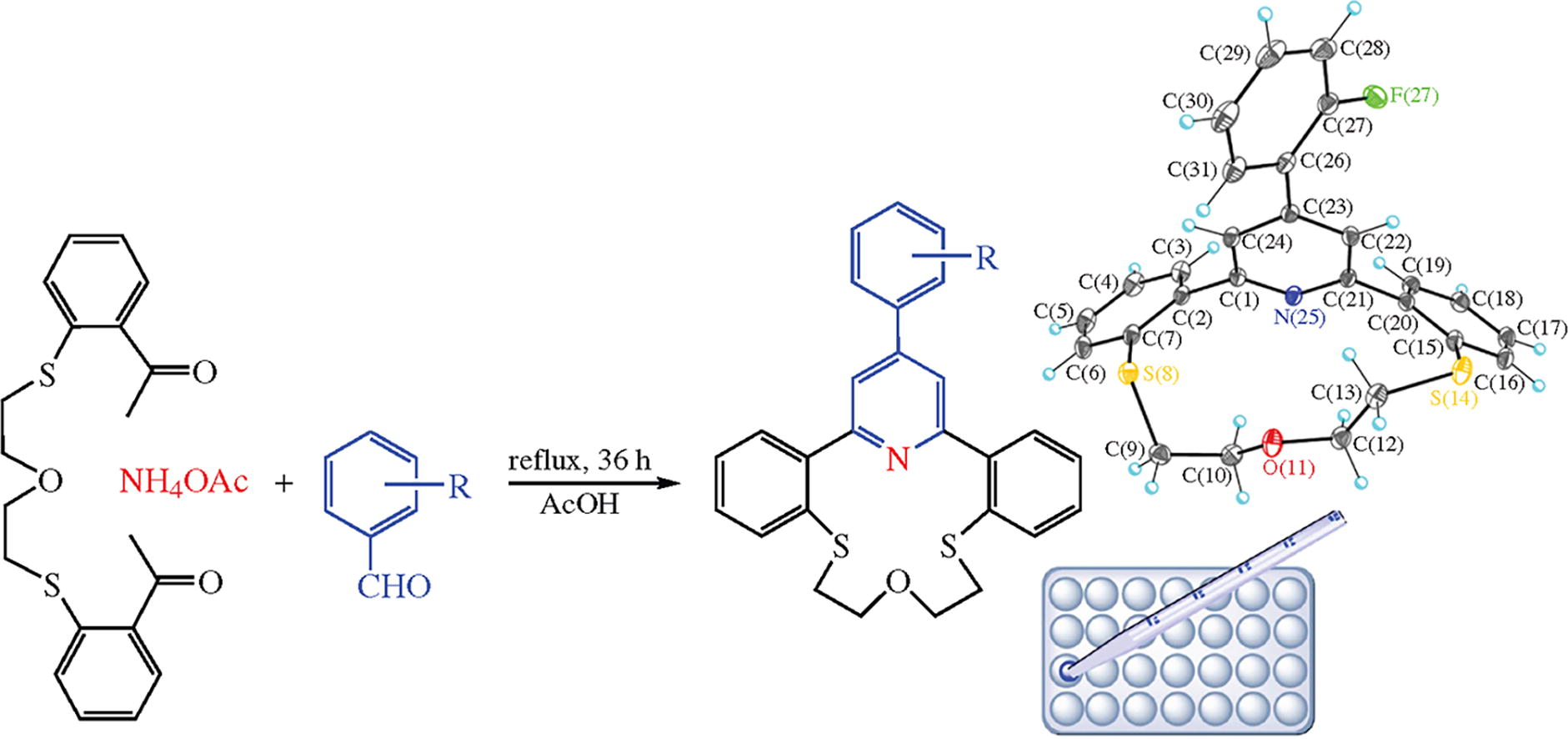
New derivatives of dithiaazacrown ethers containing ψ-arylpyridine subunits were prepared based on the Hantzsch synthesis. The obtained compounds showed potential activities toward Hep-G2 cancer cell line, in both in silico and in vitro studies. The compounds were also good ligands for complex chemistry with metal ions due to possessing up to four heteroatoms and relatively big size of the crown ring.
References
.

Krause L., Herbst-Irmer R., Sheldrick G.M., Stalke D.
Journal of Applied Crystallography,
2015
.

Levov A.N., Le An’ T., Komarova A.I., Strokina V.M., Soldatenkov A.T., Khrustalev V.N.
Russian Journal of Organic Chemistry,
2008
.
Le T.A., Truong H.H., Thi T.P., Thi N.D., To H.T., Thi H.P., Soldatenkov A.T.
Mendeleev Communications,
2015
.
![Synthesis and molecular structure of bis(benzo)aza-14-crown-4 ethers with 7-azabicyclo[3.3.1]nonane and homologous fragments](/storage/images/resized/oZgeErrVFhuDksyqFURLvYS1wtVSBWczh001igGo_small_thumb.webp)
Levov A.N., Komarov A.I., Soldatenkov A.T., Avramenko G.V., Soldatova S.A., Khrustalev V.N.
Russian Journal of Organic Chemistry,
2008
.

Morris G.M., Huey R., Lindstrom W., Sanner M.F., Belew R.K., Goodsell D.S., Olson A.J.
Journal of Computational Chemistry,
2009
.

Skehan P., Storeng R., Scudiero D., Monks A., McMahon J., Vistica D., Warren J.T., Bokesch H., Kenney S., Boyd M.R.
Journal of the National Cancer Institute,
1990
.

Qi X., Xiang H., He Q., Yang C.
Organic Letters,
2014
.

Wei H., Li Y., Xiao K., Cheng B., Wang H., Hu L., Zhai H.
Organic Letters,
2015
.

Ganjali M., Norouzi P., Rezapour M., Faridbod F., Pourjavid M.
Sensors,
2006
.

Ullah F., Khan T.A., Iltaf J., Anwar S., Khan M.F., Khan M.R., Ullah S., Fayyaz ur Rehman M., Mustaqeem M., Kotwica-Mojzych K., Mojzych M.
Applied Sciences (Switzerland),
2022
.

Wei Y., Yoshikai N.
Journal of the American Chemical Society,
2013
.

Xi L., Zhang R., Liang S., Chen S., Yu X.
Organic Letters,
2014
.

Zeng Y., Pei H., Wang Z., Yan F., Li J., Cui Z., He B.
ACS Omega,
2018
.

Yokoyama T., Mizuguchi M.
Journal of Medicinal Chemistry,
2019
.

Yoo C., Dodge H.M., Miller A.J.
Chemical Communications,
2019
.

Likhitwitayawuid K., Angerhofer C.K., Cordell G.A., Pezzuto J.M., Ruangrungsi N.
Journal of Natural Products,
1993
.

Thi T.T., Do T.T., Nguyen T.D., Luu V.B., Polyakova E.I., Thi T.V., Le T.A.
Chemistry of Heterocyclic Compounds,
2020
.
![Synthesis, structural aspects, antimicrobial activity and ion transport investigation of five new [1+1] condensed cycloheterophane peptides](/storage/images/resized/voXLqlsvTwv5p3iMQ8Dhs95nqB4AXOG7Taj7G4ra_small_thumb.webp)
Zaim Ö., Aghatabay N.M., Gürbüz M.U., Baydar Ç., Dülger B.
Journal of Inclusion Phenomena and Macrocyclic Chemistry,
2013
.

Stamos J., Sliwkowski M.X., Eigenbrot C.
Journal of Biological Chemistry,
2002
.

Cohen M.H., Johnson J.R., Chen Y., Sridhara R., Pazdur R.
Oncologist,
2005
.

Jun K., Kwon H., Park S., Lee E., Karki R., Thapa P., Lee J., Lee E., Kwon Y.
European Journal of Medicinal Chemistry,
2014
.

Karki R., Thapa P., Yoo H.Y., Kadayat T.M., Park P., Na Y., Lee E., Jeon K., Cho W., Choi H., Kwon Y., Lee E.
European Journal of Medicinal Chemistry,
2012
.

Thapa P., Karki R., Yun M., Kadayat T.M., Lee E., Kwon H.B., Na Y., Cho W., Kim N.D., Jeong B., Kwon Y., Lee E.
European Journal of Medicinal Chemistry,
2012
.

Karki R., Thapa P., Kang M.J., Jeong T.C., Nam J.M., Kim H., Na Y., Cho W., Kwon Y., Lee E.
Bioorganic and Medicinal Chemistry,
2010
.
![Synthesis and Molecular Structure of Dibenzo [4-(α-Thienyl- and α-Pyrrolyl)pyrido]aza-14-crown-4 Ethers](/storage/images/resized/PmVbwfUc2aRdrnSFGtkfcabQRXGkjjb14bQC1gcl_small_thumb.webp)
Le A.T., Truong H.H., Nguyen P.T., Pham H.T., Kotsuba V.E., Soldatenkov A.T., Khrustalev V.N., Wodajo A.T.
Macroheterocycles,
2014
.
Nguyen D.T., Truong H.H., Dao N.T., Tran V.T., Gorchakova O.S., Le A.T.
Mendeleev Communications,
2023
.
Dao N.T., Do T.T., Pham T.T., Tran V.T., Khrustalev V.N., Le A.T.
Mendeleev Communications,
2023