Keywords
aza-A-homosteroids
azidation
cytotoxicity.
isomerism
M06-2X and MP2 calculations
steroids
tetrazoles
X-ray structures
Abstract
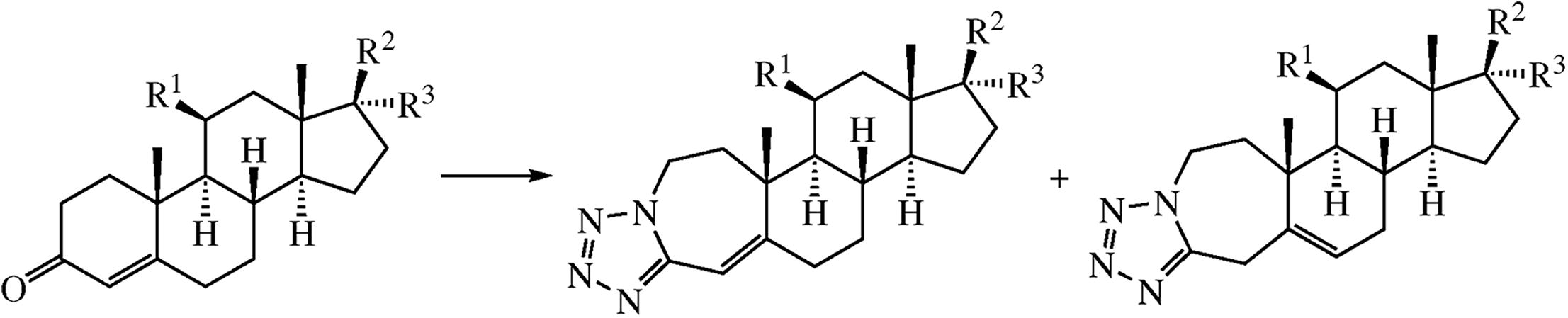
A series of new 1’H-tetrazolo[1’,5’-c]-fused 3-aza-A- homosteroids were synthesized by azidation of progesterone, testosterone and hydrocortisone acetate in the presence of silicon tetrachloride. According to NMR spectroscopy and X-ray analysis, two double bond positional isomers (double bond in ring A or in ring B) are formed in various ratios; according to quantum chemical calculations, their energies are close within 0.4 kcal mol-1. Only low cytotoxic activity against Hep-2, MCF-7, HepG2, and Hek293 cell lines was determined in vitro for the compounds obtained.
References
.

Dolomanov O.V., Bourhis L.J., Gildea R.J., Howard J.A., Puschmann H.
Journal of Applied Crystallography,
2009
.

Stulov S.V., Misharin A.Y.
Chemistry of Heterocyclic Compounds,
2013
.

Herr R.J.
Bioorganic and Medicinal Chemistry,
2002
.

Palatinus L., Chapuis G.
Journal of Applied Crystallography,
2007
.

Palatinus L., Prathapa S.J., van Smaalen S.
Journal of Applied Crystallography,
2012
.

Palatinus L., van der Lee A.
Journal of Applied Crystallography,
2008
.

Popova E.A., Trifonov R.E., Ostrovskii V.A.
Russian Chemical Reviews,
2019
.

Trifonov R.E., Ostrovskii V.A.
Russian Journal of Organic Chemistry,
2006
.
Zorina A.D., Nikiforova N.S., Zarubaev V.V., Marchenko S.A., Selivanov S.I., Starova G.L., Mehtiev A.R., Rodionov E.I., Rodionova A.A., Trifonov R.E.
Mendeleev Communications,
2019
.

Zhang L., Zhang Z., Li M., Wei Z., Jin X., Piao H.
Bioorganic and Medicinal Chemistry Letters,
2019
.

Trifonov R.E., Alkorta I., Ostrovskii V.A., Elguero J.
Journal of Molecular Structure THEOCHEM,
2004
.
![Synthesis of 2-Mono- and 2,2-Bis[2-(1H-tetrazol-5-yl)ethyl] Derivatives of Dipterocarpol](/storage/images/resized/oZgeErrVFhuDksyqFURLvYS1wtVSBWczh001igGo_small_thumb.webp)
Rodionov E.I., Kovaleva A.A., Zorina A.D., Starova G.L., Trifonov R.E.
Russian Journal of Organic Chemistry,
2018
.

Penov-Gaši K.M., Oklješa A.M., Petri E.T., Ćelić A.S., Djurendić E.A., Klisurić O.R., Csanadi J.J., Batta G., Nikolić A.R., Jakimov D.S., Sakač M.N.
MedChemComm,
2013
.

Salama T.A., El-Ahl A.S., Khalil A.M., Girges M.M., Lackner B., Steindl C., Elmorsy S.S.
Monatshefte fur Chemie,
2003
.

.

Kiss A., Herman B.E., Görbe T., Mernyák E., Molnár B., Wölfling J., Szécsi M., Schneider G.
Steroids,
2018
.
![Tetrahydropyrazolo[1,5-a]pyridine-fused steroids and their in vitro biological evaluation in prostate cancer](/storage/images/resized/GDnYOu1UpMMfMMRV6Aqle4H0YLLsraeD9IP9qScG_small_thumb.webp)
Jorda R., Lopes S.M., Řezníčková E., Ajani H., Pereira A.V., Gomes C.S., M.V.D. Pinho e Melo T.
European Journal of Medicinal Chemistry,
2019
.

Jorda R., Řezníčková E., Kiełczewska U., Maj J., Morzycki J.W., Siergiejczyk L., Bazgier V., Berka K., Rárová L., Wojtkielewicz A.
European Journal of Medicinal Chemistry,
2019
.

Birudukota N., Mudgal M.M., Shanbhag V.
Steroids,
2019
.

Sloan K.B., Bodor N., Little R.J.
Tetrahedron,
1981
.

Škorić D.Đ., Klisurić O.R., Jakimov D.S., Sakač M.N., Csanádi J.J.
Beilstein Journal of Organic Chemistry,
2021
.

Kaplanskiy M.V., Faizullina O.E., Trifonov R.E.
Journal of Physical Chemistry A,
2023
.

Trifonov R.E., Ostrovskii V.A.
International Journal of Molecular Sciences,
2023