Keywords
abiraterone prodrugs
citric acid
cytochrome P450 17A1 inhibitors
depot formulations
pimelic acid
plasma hydrolysis
prostate cancer.
steroid derivatives
succinic acid
twin drugs
Abstract
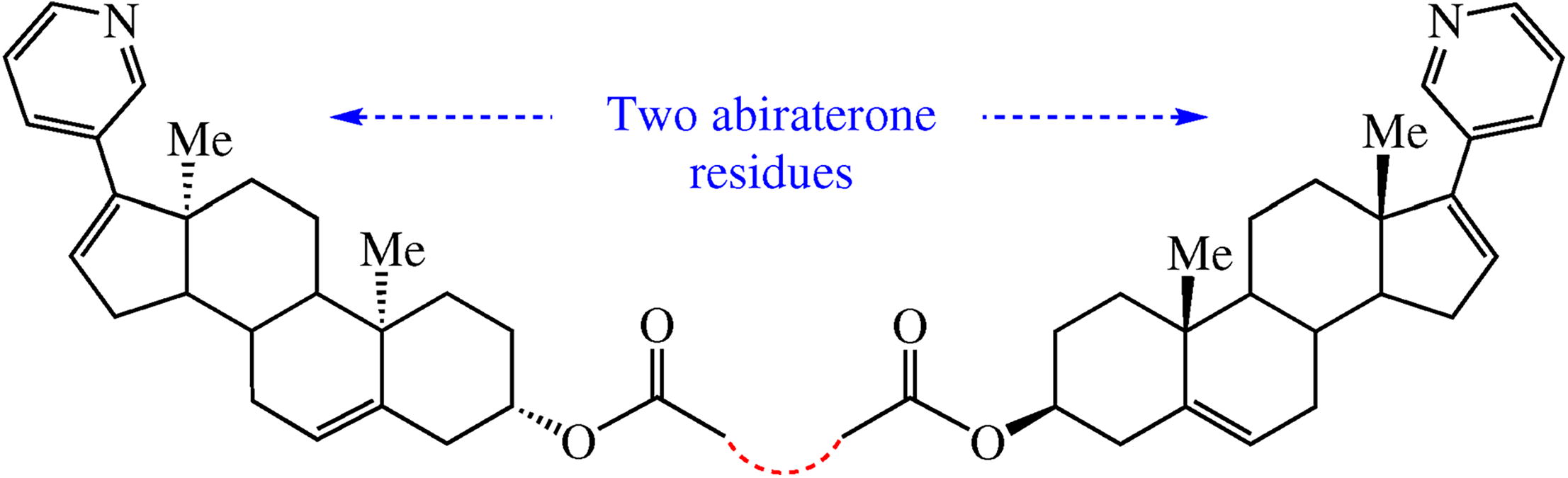
The synthesis of homodimeric bis-esters of abiraterone (an inhibitor of androgens synthesis used for treatment of prostate cancer) as a novel depot form of the drug intended for parenteral administration has been undertaken. The conjugate with succinic linker has been synthesized, while only mono-esters of malic and citric acid were obtained. The target ‘twin’ demonstrated excellent characteristics in preliminary biotests, proving the concept of long-lasting depot-prodrug.
References
.
Nurieva E.V., Alexeev A.A., Zefirov N.A., Milaeva E.R., Kovaleva N.V., Proshin A.N., Makhaeva G.F., Zefirova O.N.
Mendeleev Communications,
2023
.

Daina A., Michielin O., Zoete V.
Scientific Reports,
2017
.

Nortcliffe A., Fleming I.N., Botting N.P., O'Hagan D.
Tetrahedron,
2014
.

Vasaitis T.S., Bruno R.D., Njar V.C.
Journal of Steroid Biochemistry and Molecular Biology,
2011
.

Liesen G.P., Sukenik C.N.
Journal of Organic Chemistry,
1987
.

Moore W.R., Sharp M., Bell C., Freeman S., Parr A., Eisner J.R., Schotzinger R.
Journal of Clinical Oncology,
2021
.

Date T., Paul K., Singh N., Jain S.
AAPS PharmSciTech,
2019
.

DeVore N.M., Scott E.E.
Nature,
2012
.

Stappaerts J., Geboers S., Snoeys J., Brouwers J., Tack J., Annaert P., Augustijns P.
European Journal of Pharmaceutics and Biopharmaceutics,
2015
.

Di L., Kerns E.H., Hong Y., Chen H.
International Journal of Pharmaceutics,
2005
.

Repta A.J., Higuchi T.
Journal of Pharmaceutical Sciences,
1969
.

Caffo O., Veccia A., Kinspergher S., Maines F.
Future Oncology,
2018
.
Zefirov N.A., Glaßl A., Radchenko E.V., Borovik A.N., Stanishevskiy V.V., Milaeva E.R., Kuznetsov S.A., Zefirova O.N.
Mendeleev Communications,
2022
.

Sedenkova K.N., Leschukov D.N., Grishin Y.K., Zefirov N.A., Gracheva Y.A., Skvortsov D.A., Hrytseniuk Y.S., Vasilyeva L.A., Spirkova E.A., Shevtsov P.N., Shevtsova E.F., Lukmanova A.R., Spiridonov V.V., Markova A.A., Nguyen M.T., et. al.
Pharmaceuticals,
2023
.

Burmistrov V.V., Morisseau C., Danilov D.V., Gladkikh B.P., D’yachenko V.S., Zefirov N.A., Zefirova O.N., Butov G.M., Hammock B.D.
Journal of Enzyme Inhibition and Medicinal Chemistry,
2023