Keywords
caproic acid
extractant
leaching of metals
microemulsion
nanostructured media.
non-ferrous metals
sodium dodecyl sulfate
Abstract
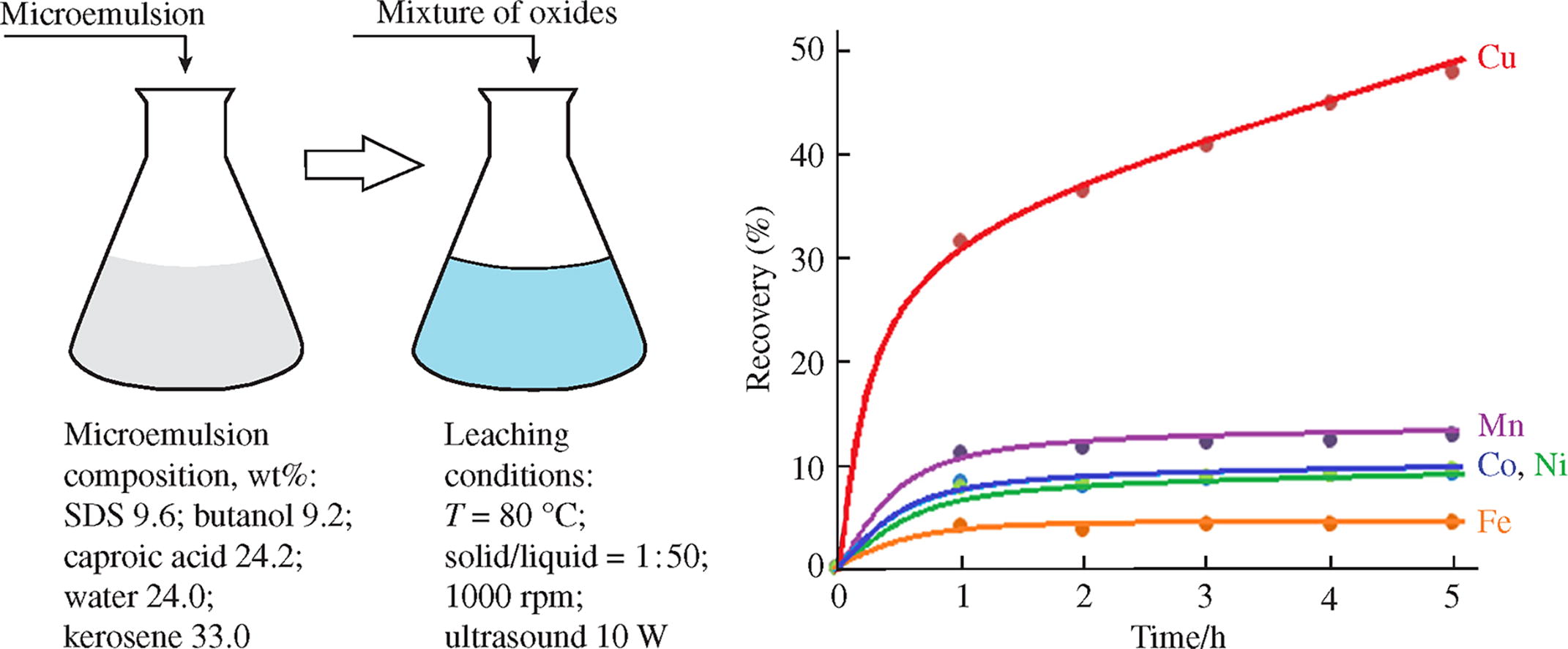
The possibility of using reverse microemulsion based on sodium dodecyl sulfate containing an extractant caproic acid for the selective recovery of non-ferrous metals and their separation from iron in the process of microemulsion leaching has been demonstrated. Using a model system with a mixture of oxides, the following recovery factors of metals are achieved in 5 h of leaching (%): Cu, 47.6; Mn, 12.8; Ni, 9.4; Co, 9.1; Fe, 4.3. When leaching from a sample of oxidized cobalt-copper concentrate, the recovery of metals is as follows (%): Cu, 60.0; Co, 51.3; Mn, 16.5; Fe, 10.0; Ni, 9.3.
References
.

Smith E.L., Abbott A.P., Ryder K.S.
Chemical Reviews,
2014
.

Pletnev I.V., Smirnova S.V., Sharov A.V., Zolotov Y.A.
Russian Chemical Reviews,
2021
.

Riaño S., Petranikova M., Onghena B., Vander Hoogerstraete T., Banerjee D., Foreman M.R., Ekberg C., Binnemans K.
RSC Advances,
2017
.

Carlesi C., Harris R.C., Abbott A.P., Jenkin G.R.
Minerals,
2022
.
Guo Y., Li H., Yuan Y., Huang J., Diao J., Li G., Xie B.
International Journal of Minerals, Metallurgy and Materials,
2021
.

Yurtov E.V., Murashova N.M.
Theoretical Foundations of Chemical Engineering,
2011
.

Polyakova A.S., Murashova N.M., Yurtov E.V.
Russian Journal of Applied Chemistry,
2020
.

Murashova N.M., Polyakova A.S., Yurtov E.V.
Colloid Journal,
2018
.

Murashova N.M., Yurtov E.V.
Theoretical Foundations of Chemical Engineering,
2022
.

Zhou N., Wu J., Yu Z., Neuman R.D., Wang D., Xu G.
Science in China Series B Chemistry,
1997
.

Murashova N.M., Levchishin S.Y., Yurtov E.V.
Hydrometallurgy,
2018
.

Jalali-Jivan M., Garavand F., Jafari S.M.
Advances in Colloid and Interface Science,
2020
.

Guo Y., Li H., Shen S., Wang C., Diao J., Xie B.
Hydrometallurgy,
2020
.
Rakshit A.K., Naskar B., Moulik S.P.
Current Science,
2019
.

Qi W., He J., Li M., Zhai M., Zhao L.
Separation and Purification Technology,
2022