Keywords
2
6-diazaspiro[3.4]octane.
antibiotic resistance
carboxamides
ESKAPE pathogens
isosteric replacement
methicillin-resistant bacteria
non-β-lactam antibiotics
organofluorine compounds
Abstract
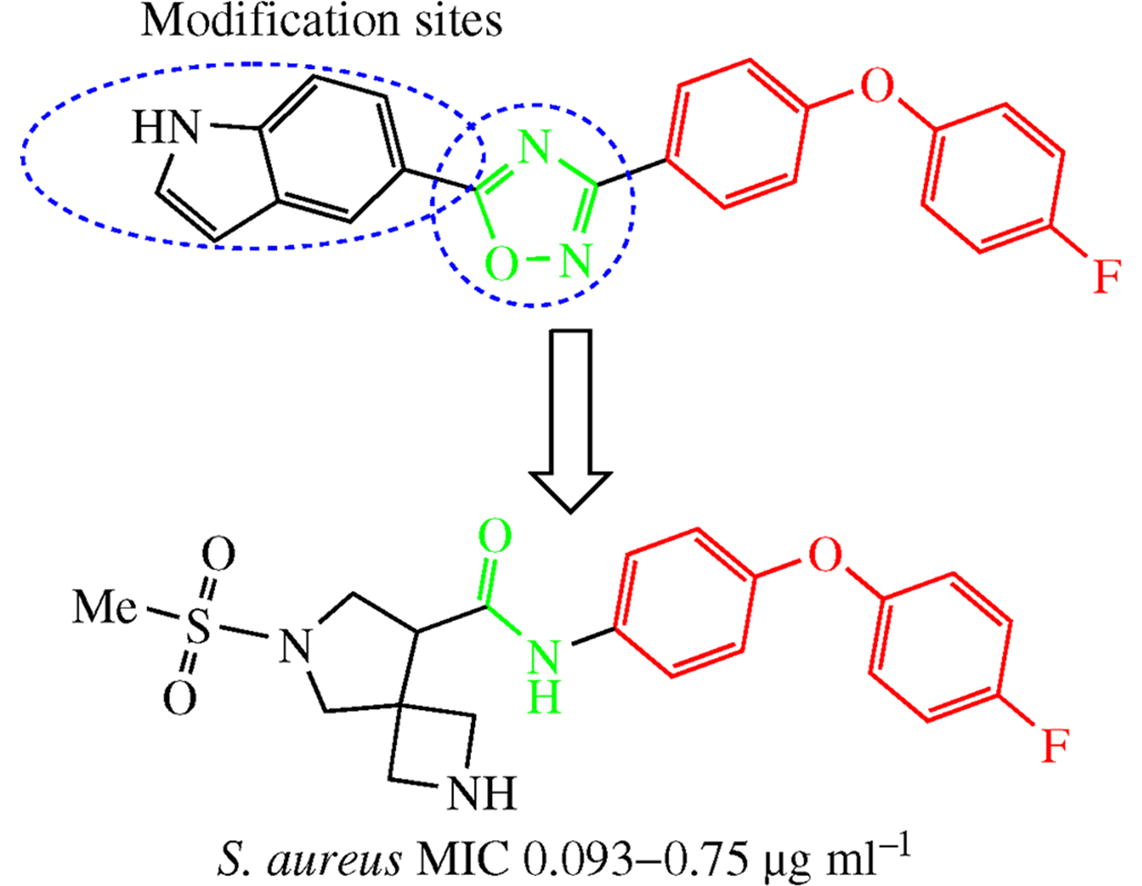
Isosteric replacement of the oxadiazole ring by amide bond in the structure of new non-β-lactam antibiotics led to compounds with higher activity against Gram-positive pathogens of ESKAPE panel. A series of 17 compounds were synthesized by acylation of 4-(4-fluorophenoxy)aniline with various amino acids. The spirocyclic derivative with 6-methylsulfonyl-2,6-diazaspiro[3.4]octane moiety showed excellent minimum inhibitory concentrations of 0.093-0.75 μg ml−1 against a number of methicillin-resistant Staphylococcus aureus strains.
References
.
Mekky A.E., Sanad S.M., Abdelfattah A.M.
Mendeleev Communications,
2022
.

O’Daniel P.I., Peng Z., Pi H., Testero S.A., Ding D., Spink E., Leemans E., Boudreau M.A., Yamaguchi T., Schroeder V.A., Wolter W.R., Llarrull L.I., Song W., Lastochkin E., Kumarasiri M., et. al.
Journal of the American Chemical Society,
2014
.

Pendleton J.N., Gorman S.P., Gilmore B.F.
Expert Review of Anti-Infective Therapy,
2013
.

Spink E., Ding D., Peng Z., Boudreau M.A., Leemans E., Lastochkin E., Song W., Lichtenwalter K., O’Daniel P.I., Testero S.A., Pi H., Schroeder V.A., Wolter W.R., Antunes N.T., Suckow M.A., et. al.
Journal of Medicinal Chemistry,
2015
.

Shalaby M.W., Dokla E.M., Serya R.A., Abouzid K.A.
European Journal of Medicinal Chemistry,
2020
.

Boudreau M.A., Ding D., Meisel J.E., Janardhanan J., Spink E., Peng Z., Qian Y., Yamaguchi T., Testero S.A., O’Daniel P.I., Leemans E., Lastochkin E., Song W., Schroeder V.A., Wolter W.R., et. al.
ACS Medicinal Chemistry Letters,
2019
.

Buommino E., De Marino S., Sciarretta M., Piccolo M., Festa C., D’Auria M.V.
Antibiotics,
2021
.

Naclerio G.A., Abutaleb N.S., Onyedibe K.I., Karanja C., Eldesouky H.E., Liang H., Dieterly A., Aryal U.K., Lyle T., Seleem M.N., Sintim H.O.
Journal of Medicinal Chemistry,
2022
.

Peacock S.J., Paterson G.K.
Annual Review of Biochemistry,
2015
.

Tresse C., Radigue R., Gomes Von Borowski R., Thepaut M., Hanh Le H., Demay F., Georgeault S., Dhalluin A., Trautwetter A., Ermel G., Blanco C., van de Weghe P., Jean M., Giard J., Gillet R., et. al.
Bioorganic and Medicinal Chemistry,
2019
.

Verma S.K., Verma R., Kumar K.S., Banjare L., Shaik A.B., Bhandare R.R., Rakesh K.P., Rangappa K.S.
European Journal of Medicinal Chemistry,
2021
.

Palavecino E.L.
Methods in Molecular Biology,
2019
.

Vedekhina T.S., Chudinov M.V., Lukin A.Y.
Fine Chemical Technologies,
2023
.

Rogacheva E., Kraeva L., Lukin A., Vinogradova L., Komarova K., Chudinov M., Gureev M., Chupakhin E.
Molecules,
2023
.

Qian Y., Birhanu B.T., Yang J., Ding D., Janardhanan J., Mobashery S., Chang M.
Journal of Medicinal Chemistry,
2023
.

Vedekhina T.S., Dogonadze M.Z., Vinogradova T.I., Chudinov M.V., Lukin A.Y.
Pharmaceutical Chemistry Journal,
2023