Keywords
3
7-diazabicyclo[3.3.1]nonanes
allosteric modulators
AMPA receptor
PAMs
patch clamp.
piperonylic acid
Abstract
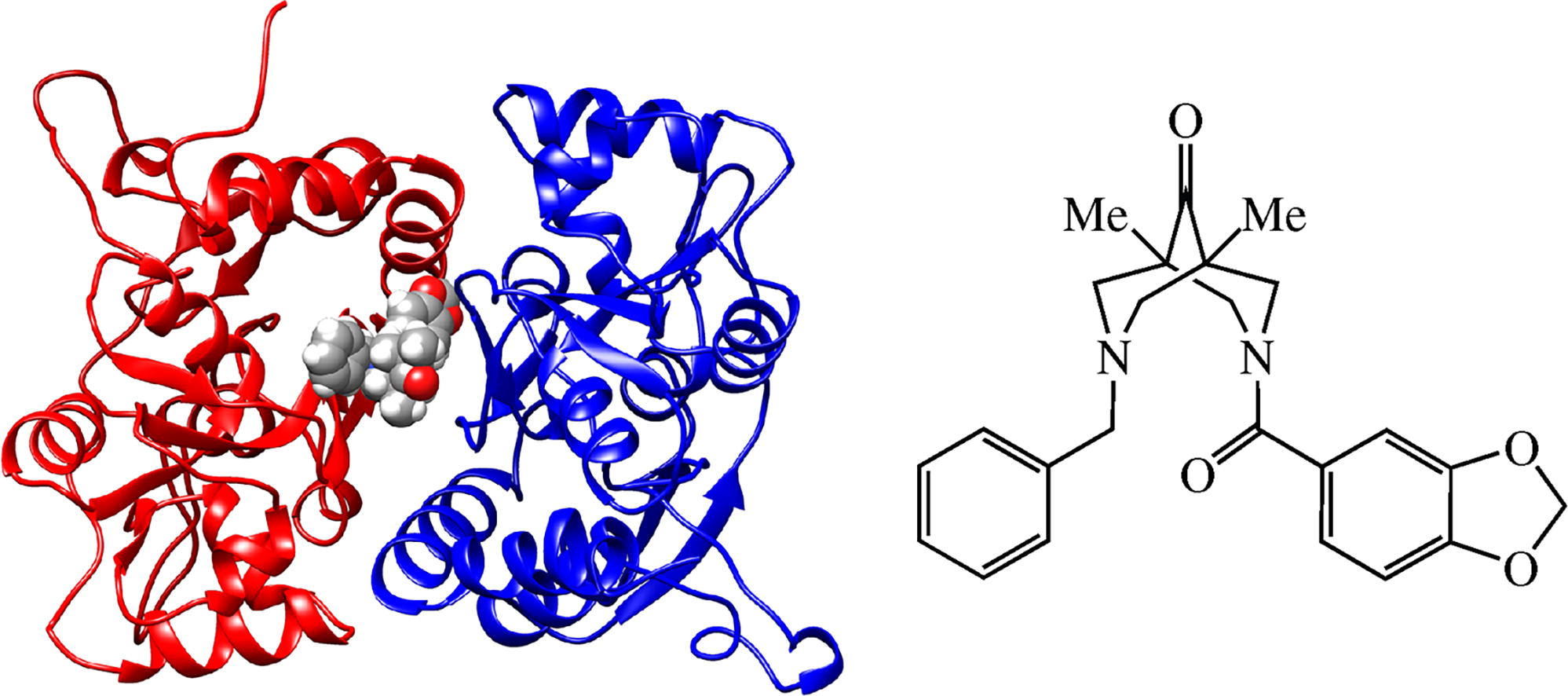
The title compound has been synthesized and assessed in vitro by means of electrophysiological patch clamp technique revealing a positive modulatory effect on the kainate-induced currents in Purkinje neurons in a wide concentration range from 10-12 to 10-6 m. Molecular docking and molecular dynamics simulation revealed a putative binding mode of this compound in the binding site of positive allosteric modulators of AMPA receptors.
References
.

Trott O., Olson A.J.
Journal of Computational Chemistry,
2009
.

Huang J., MacKerell A.D.
Journal of Computational Chemistry,
2013
.

Abraham M.J., Murtola T., Schulz R., Páll S., Smith J.C., Hess B., Lindahl E.
SoftwareX,
2015
.

Vanommeslaeghe K., Hatcher E., Acharya C., Kundu S., Zhong S., Shim J., Darian E., Guvench O., Lopes P., Vorobyov I., Mackerell A.D.
Journal of Computational Chemistry,
2009
.

Reuillon T., E. Ward S., Beswick P.
Current Topics in Medicinal Chemistry,
2016
.
Lavrov M.I., Veremeeva P.N., Golubeva E.A., Radchenko E.V., Zamoyski V.L., Grigoriev V.V., Palyulin V.A.
Mendeleev Communications,
2022
.

Radchenko E.V., Dyabina A.S., Palyulin V.A.
Molecules,
2020
.

Bickerton G.R., Paolini G.V., Besnard J., Muresan S., Hopkins A.L.
Nature Chemistry,
2012
.

Sushko I., Novotarskyi S., Körner R., Pandey A.K., Rupp M., Teetz W., Brandmaier S., Abdelaziz A., Prokopenko V.V., Tanchuk V.Y., Todeschini R., Varnek A., Marcou G., Ertl P., Potemkin V., et. al.
Journal of Computer-Aided Molecular Design,
2011
.

Radchenko E.V., Rulev Y.A., Safanyaev A.Y., Palyulin V.A., Zefirov N.S.
Doklady Biochemistry and Biophysics,
2017
.

Radchenko E.V., Dyabina A.S., Palyulin V.A., Zefirov N.S.
Russian Chemical Bulletin,
2016
.
![Novel Positive Allosteric Modulators of AMPA Receptors Based on 3,7-Diazabicyclo[3.3.1]nonane Scaffold](/storage/images/resized/voXLqlsvTwv5p3iMQ8Dhs95nqB4AXOG7Taj7G4ra_small_thumb.webp)
Lavrov M.I., Karlov D.S., Voronina T.A., Grigoriev V.V., Ustyugov A.A., Bachurin S.O., Palyulin V.A.
Molecular Neurobiology,
2019
.

Lee K., Goodman L., Fourie C., Schenk S., Leitch B., Montgomery J.M.
Advances in Protein Chemistry and Structural Biology,
2016
.

Golubeva E.A., Lavrov M.I., Radchenko E.V., Palyulin V.A.
Biomolecules,
2022
.
Hansen K.B., Wollmuth L.P., Bowie D., Furukawa H., Menniti F.S., Sobolevsky A.I., Swanson G.T., Swanger S.A., Greger I.H., Nakagawa T., McBain C.J., Jayaraman V., Low C., Dell’Acqua M.L., Diamond J.S., et. al.
Pharmacological Reviews,
2021
.

Goffin E., Fraikin P., Abboud D., de Tullio P., Beaufour C., Botez I., Hanson J., Danober L., Francotte P., Pirotte B.
European Journal of Medicinal Chemistry,
2023
.
![New stereoselective intramolecular redox reaction in the system of 3,7-diazabicyclo[3.3.1]nonan-9-one](/storage/images/resized/oZgeErrVFhuDksyqFURLvYS1wtVSBWczh001igGo_small_thumb.webp)
Vatsadze S.Z., Tyurin V.S., Zatsman A.I., Manaenkova M.A., Semashko V.S., Krut’ko D.P., Zyk N.V., Churakov A.V., Kuz’mina L.G.
Russian Journal of Organic Chemistry,
2006
.

Kuznetsov A.I., Basargin E.B., Moskovkin A.S., Ba M.K., Miroshnichenko I.V., Botnikov M.Y., Unkovskii B.V.
Chemistry of Heterocyclic Compounds,
1985
.

Ward S.E., Harries M.H., Aldegheri L., Bradford A.M., Ballini E., Dawson L., Lacroix L., Pardoe J., Starr K., Weil A., Waters K., Atack J.R., Woolley M.
Journal of Psychopharmacology,
2019
.

Zhao Y., Chen S., Swensen A.C., Qian W., Gouaux E.
Science,
2019
.

Gautam V., Gaurav A., Masand N., Lee V.S., Patil V.M.
Molecular Diversity,
2022
.

Structural and Functional Architecture of AMPA-Type Glutamate Receptors and Their Auxiliary Proteins
Greger I.H., Watson J.F., Cull-Candy S.G.
Neuron,
2017
.

Danon J.J., Reekie T.A., Kassiou M.
Trends in Chemistry,
2019
.

Matthews P.M., Pinggera A., Kampjut D., Greger I.H.
Neuropharmacology,
2021
.
Golubeva E.A., Lavrov M.I., Veremeeva P.N., Bovina E.M., Radchenko E.V., Topchiy M.A., Asachenko A.F., Zamoyski V.L., Grigoriev V.V., Palyulin V.A.
Mendeleev Communications,
2023
.

Golubeva E.A., Lavrov M.I., Veremeeva P.N., Vyunova T.V., Shevchenko K.V., Topchiy M.A., Asachenko A.F., Palyulin V.A.
International Journal of Molecular Sciences,
2023