Keywords
2-a]benzimidazol-9-ium salts
aminoazoles
Cyclization
imidazo[1
iodoacetyl azoles
quantum chemical calculations.
transamination
Abstract
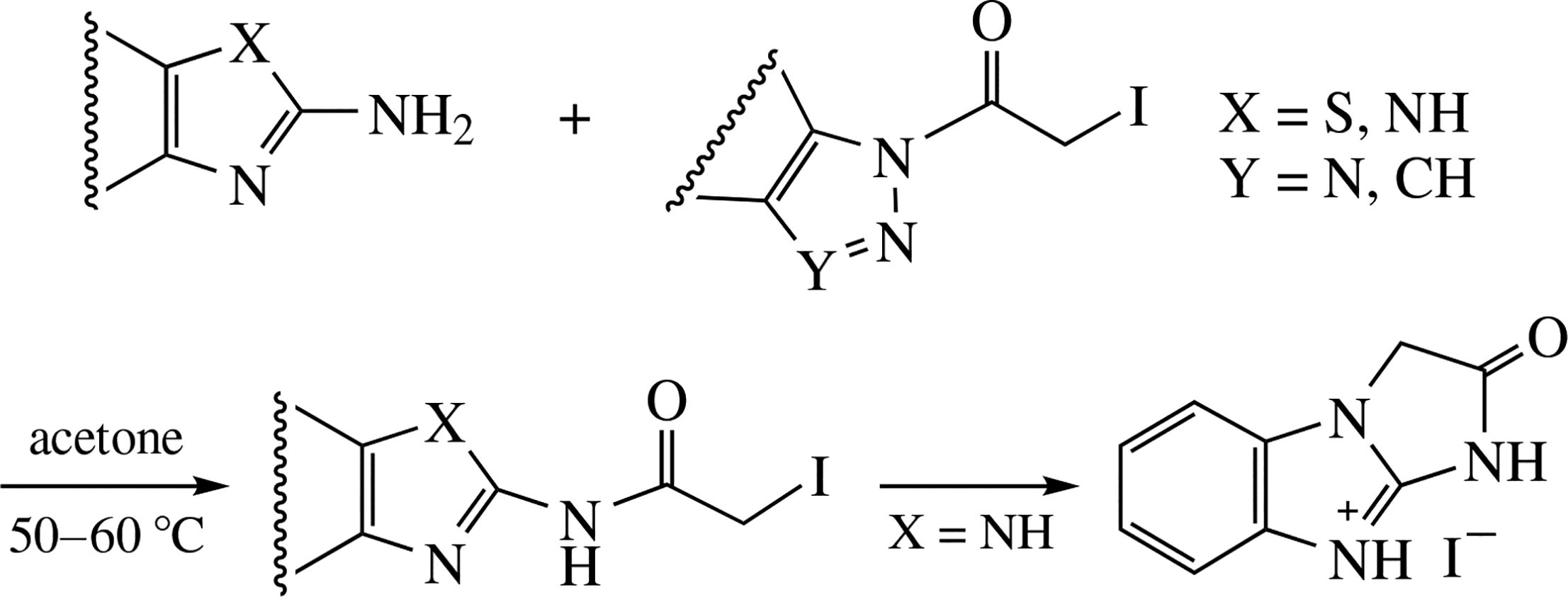
N-Iodoacetyl-substituted azoles undergo transfer of iodoacetyl group from heterocyclic N atom toward the amino group of amino azoles, unlike the earlier investigated iodomethyl ketones. The proposed mechanism explaining the observed difference in such transamination is confirmed by calculations. The product derived from 2-aminobenzimidazole is cyclized into fused imidazo[1,2-α]benzimidazol- 9-ium salt.
References
.

Sivaev I.B.
Chemistry of Heterocyclic Compounds,
2017
.

Zhilitskaya L.V., Shainyan B.A., Yarosh N.O.
Molecules,
2021
.

Demir Özkay Ü., Kaya C., Acar Çevik U., Can Ö.
Molecules,
2017
.

Abo-Dya N.E., Biswas S., Basak A., Avan I., Alamry K.A., Katritzky A.R.
Journal of Organic Chemistry,
2013
.
![Synthesis of novel derivatives of 7,8-dihydro-6H -imidazo[2,1-b ][1,3]benzothiazol-5-one and their virus-inhibiting activity against influenza A virus](/storage/images/resized/bRyGpdm98BkAUYiK1YFNpl5Z7hPu6Gd87gbIeuG3_small_thumb.webp)
Galochkina A.V., Bollikanda R.K., Zarubaev V.V., Tentler D.G., Lavrenteva I.N., Slita A.V., Chirra N., Kantevari S.
Archiv der Pharmazie,
2018
.

Sultanova R.M., Lobov A.N., Shumadalova A.V., Meshcheryakova S.A., Zileeva Z.R., Khusnutdinova N.S., Vakhitov V.A., Vakhitova Y.V.
Natural Product Research,
2019
.

Zhilitskaya L.V., Shagun L.G., Dorofeev I.A., Yarosh N.O., Larina L.I.
Russian Journal of Organic Chemistry,
2018
.

Srinivasarao S., Nandikolla A., Nizalapur S., Yu T.T., Pulya S., Ghosh B., Murugesan S., Kumar N., Chandra Sekhar K.V.
RSC Advances,
2019
.

Ibrahim D.A., Lasheen D.S., Zaky M.Y., Ibrahim A.W., Vullo D., Ceruso M., Supuran C.T., Abou El Ella D.A.
Bioorganic and Medicinal Chemistry,
2015
.

Demir Özkay Ü., Can Ö.D., Sağlık B.N., Acar Çevik U., Levent S., Özkay Y., Ilgın S., Atlı Ö.
Bioorganic and Medicinal Chemistry Letters,
2016
.
![Antioxidant activity of novel imidazo[2,1-b]thiazole derivatives: Design, synthesis, biological evaluation, molecular docking study and in silico ADME prediction](/storage/images/resized/GDnYOu1UpMMfMMRV6Aqle4H0YLLsraeD9IP9qScG_small_thumb.webp)
Dincel E.D., Gürsoy E., Yilmaz-Ozden T., Ulusoy-Güzeldemirci N.
Bioorganic Chemistry,
2020
.
![Evaluation of imidazo[2,1–b]thiazole-based anticancer agents in one decade (2011–2020): Current status and future prospects](/storage/images/resized/GDnYOu1UpMMfMMRV6Aqle4H0YLLsraeD9IP9qScG_small_thumb.webp)
Sbenati R.M., Semreen M.H., Semreen A.M., Shehata M.K., Alsaghir F.M., El-Gamal M.I.
Bioorganic and Medicinal Chemistry,
2021
.
![Design, biological evaluation, molecular docking study and in silico ADME prediction of novel imidazo[2,1-b]thiazole derivatives as a novel class of α-glucosidase inhibitors](/storage/images/resized/GDnYOu1UpMMfMMRV6Aqle4H0YLLsraeD9IP9qScG_small_thumb.webp)
Dincel E.D., Hasbal-Celikok G., Yilmaz-Ozden T., Ulusoy-Güzeldemirci N.
Journal of Molecular Structure,
2021
.

Rezaei Z., Sarkari B., Khabnadideh S., Farjami M., Mehrjou M., Yazdi A., Riazimontazer E., Fararouei M.
Anti-Infective Agents,
2019
.

Dorofeev I.A., Zhilitskaya L.V., Yarosh N.O., Shainyan B.A.
Molecules,
2023
.
![Design, synthesis, biological evaluation, molecular docking, and dynamic simulation study of novel imidazo[2,1-b]thiazole derivatives as potent antioxidant agents](/storage/images/resized/GDnYOu1UpMMfMMRV6Aqle4H0YLLsraeD9IP9qScG_small_thumb.webp)
Dincel E.D., Hasbal-Celikok G., Yilmaz-Ozden T., Ulusoy-Güzeldemirci N.
Journal of Molecular Structure,
2022
.

Coşkun G.P., Sahin Z., Erdoğan Ö., Çevik Ö., Biltekin S.N., Yurttas L., Berk B., Ülgen M., Demirayak Ş.
Journal of Molecular Structure,
2023
.