Keywords
1-a]iso-quinoline
alkaloids
homoveratrylamine
isoquinoline
N-(homoveratryl)maleimide
phosphorus ylide
Pictet–Spengler reaction.
tetrahydropyrrolo[2
Abstract
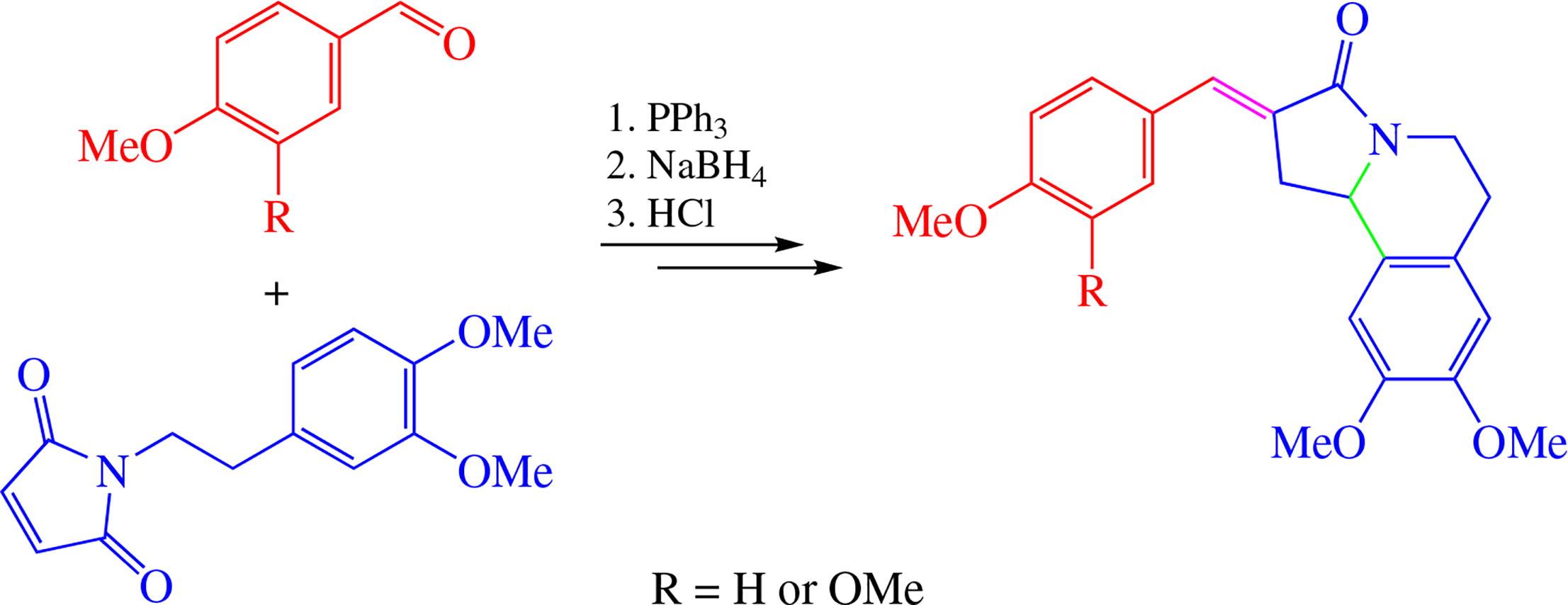
A simple and convenient synthesis of new isoquinolinone derivatives based on N-(homoveratryl)succinimide phosphorus ylide and natural aldehydes is suggested. The final formation of the tetrahydropyrrolo[2,1-a]isoquinoline framework occurs via the Pictet-Spengler cyclization.
References
.
Zubenko A.A., Morkovnik A.S., Divaeva L.N., Sochnev V.S., Demidov O.P., Klimenko A.I., Fetisov L.N., Bodryakov A.N., Bodryakova M.A., Borodkin G.S.
Mendeleev Communications,
2022
.
Sochnev V.S., Morkovnik A.S., Zubenko A.A., Divaeva L.N., Demidov O.P., Gribanova T.N., Fetisov L.N., Chekrysheva V.V., Kononenko K.N., Bodryakova M.A., Klimenko A.I., Borodkin G.S., Estrin I.A.
Mendeleev Communications,
2022
.

Panchaud P., Bruyère T., Blumstein A., Bur D., Chambovey A., Ertel E.A., Gude M., Hubschwerlen C., Jacob L., Kimmerlin T., Pfeifer T., Prade L., Seiler P., Ritz D., Rueedi G., et. al.
Journal of Medicinal Chemistry,
2017
.

Meiring L., Petzer J.P., Petzer A.
Mini-Reviews in Medicinal Chemistry,
2017
.

Zhang Q., Tu G., Zhao Y., Cheng T.
Tetrahedron,
2002
.

Calcaterra A., Mangiardi L., Delle Monache G., Quaglio D., Balducci S., Berardozzi S., Iazzetti A., Franzini R., Botta B., Ghirga F.
Molecules,
2020
.

Karanja C.W., Naganna N., Abutaleb N.S., Dayal N., Onyedibe K.I., Aryal U., Seleem M.N., Sintim H.O.
Molecules,
2022
.

Crestey F., Jensen A.A., Borch M., Andreasen J.T., Andersen J., Balle T., Kristensen J.L.
Journal of Medicinal Chemistry,
2013
.

Chrzanowska M., Grajewska A., Rozwadowska M.D.
Chemical Reviews,
2016
.

Schwan J., Kleoff M., Hartmayer B., Heretsch P., Christmann M.
Organic Letters,
2018
.
![Multicomponent Reaction for the Synthesis of 5,6-Dihydropyrrolo[2,1-a]isoquinolines](/storage/images/resized/iLiQsFqFaSEx6chlGQ5fbAwF6VYU3WWa08hkss0g_small_thumb.webp)
Ghosh A., Kolle S., Barak D.S., Kant R., Batra S.
ACS Omega,
2019
.

Habel D., Nair D.S., Kallingathodi Z., Mohan C., Pillai S.M., Nair R.R., Thomas G., Haleema S., Gopinath C., Abdul R.V., Fritz M., Puente A.R., Johnson J.L., Polavarapu P.L., Ibnusaud I., et. al.
Journal of Natural Products,
2020
.

Qi C., Guo T., Xiong W., Wang L., Jiang H.
ChemistrySelect,
2017
.

Xu W., Tang L., Ge C., Chen J., Zhou L.
Advanced Synthesis and Catalysis,
2019
.

Wu L., Hao Y., Liu Y., Wang Q.
Organic and Biomolecular Chemistry,
2019
.

Sakhautdinova G.F., Sakhautdinov I.M., Mustafin A.G., Yunusov M.S.
Chemistry of Natural Compounds,
2022
.

Sakhautdinova G.F., Sakhautdinov I.M., Nazarov I.S., Mustafin A.G., Vinogradova V.I., Yunusov M.S.
Chemistry of Natural Compounds,
2022
.

Aly A.A., El-Sheref E.M., Mourad A.E., Bakheet M.E., Bräse S.
Molecular Diversity,
2019
.
![Functionalized organolithium compounds: Generation via reductive lithiation and nucleophilic addition to N-phenethylimides. Access to functionalized dihydropyrrolo[2,1-a]isoquinolinones](/storage/images/resized/GDnYOu1UpMMfMMRV6Aqle4H0YLLsraeD9IP9qScG_small_thumb.webp)
Manteca I., Etxarri B., Ardeo A., Arrasate S., Osante I., Sotomayor N., Lete E.
Tetrahedron,
1998
.
![A Short and Efficient Approach to Pyrrolo[2,1-a]isoquinoline and Pyrrolo[2,1-a]benzazepine Derivatives](/storage/images/resized/xqixcltwJYe6H8Uco2JbAFfIOzt7UNKH0OcPOPzO_small_thumb.webp)
Lebreton J., Mathé-Allainmat M., Amri H., Jebali K., Planchat A.
Synthesis,
2016
.

Gao F., Liu H., Li L., Guo J., Wang Y., Zhao M., Peng S.
Bioorganic and Medicinal Chemistry Letters,
2015
.

Chang C., Huang C., Huang Y., Lin K., Lee Y., Wang C.
Synthetic Communications,
2010
.

Zheng M., Yang Y., Zhao M., Zhang X., Wu J., Chen G., Peng L., Wang Y., Peng S.
European Journal of Medicinal Chemistry,
2011
.

Pieper P., McHugh E., Amaral M., Tempone A.G., Anderson E.A.
Tetrahedron,
2020
.

Rao L.B., Sreenivasulu C., Kishore D.R., Satyanarayana G.
Tetrahedron,
2022
.

Reddy T.R., Reddy D.N., Reddy B.K., Kasturaiah C., Yadagiri K.
Asian Journal of Chemistry,
2018