Keywords
antibacterial compounds
antibiotics potentiation
bCSE
benzo[b]thiophenes
cross-coupling.
indole-based inhibitors
indoles
NL3
Abstract
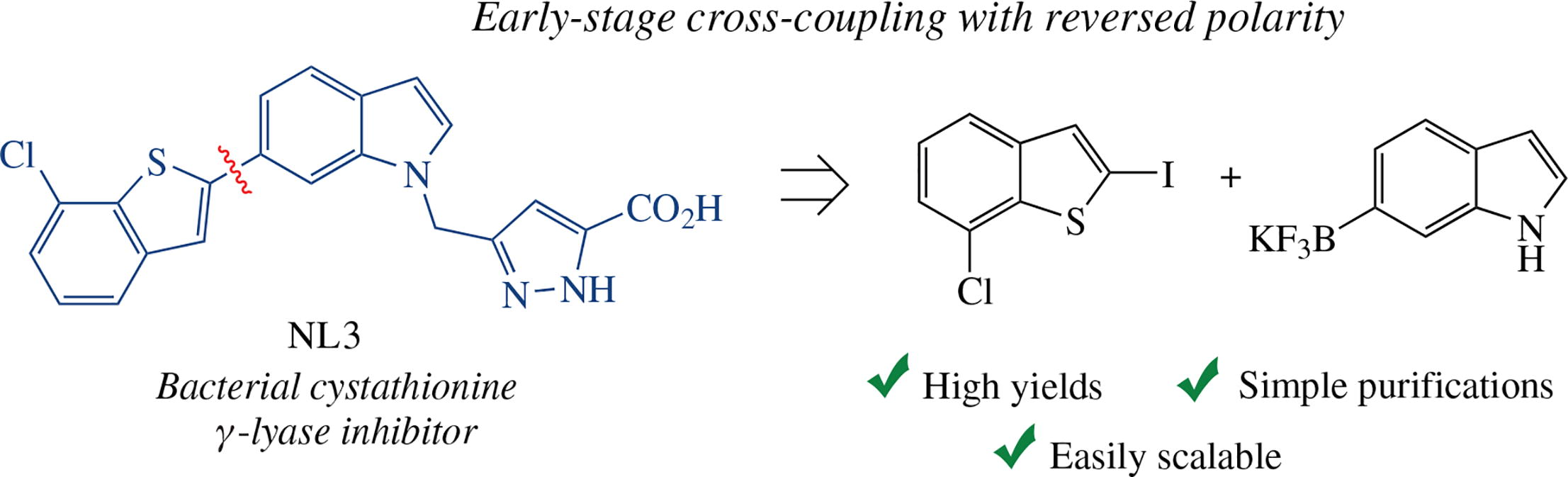
A convenient and efficient synthesis of 3-{[6-(7-chloro- benzo[b]thiophen-2-yl)-1H-indol-1-yl]methyl}-1H-pyrazole- 5-carboxylic acid (NL3), which is currently among the most active and promising bacterial cystathionine γ-lyase (bCSE) inhibitors, has been developed. It is based on shifting the key stage of [Pd]-catalyzed cross-coupling of the indole and benzothiophene counterparts to the beginning of the synthetic scheme, with the polarity reversal of the components being coupled, to give 6-(7-chlorobenzo[b]thiophen-2-yl)-1H- indole as the key intermediate. The STD NMR method was used to estimate the NL3 compound obtained in the optimized synthesis as a ligand to saCSE (the main producer of H2S in pathogenic S. aureus).
References
.

Mayer M., Meyer B.
Angewandte Chemie - International Edition,
1999
.

Martin R., Buchwald S.L.
Accounts of Chemical Research,
2008
.

Angulo J., Enríquez-Navas P., Nieto P.
Chemistry - A European Journal,
2010
.

Hwang T.L., Shaka A.J.
Journal of Magnetic Resonance Series A,
1995
.

Prieto M., Zurita E., Rosa E., Muñoz L., Lloyd-Williams P., Giralt E.
Journal of Organic Chemistry,
2004
.

Sagong H., Kim B., Joo S., Kim K.
Journal of Agricultural and Food Chemistry,
2020
.

Jiang P., Che X., Liao Y., Huang H., Deng G.
RSC Advances,
2016
.

Shatalin K., Nuthanakanti A., Kaushik A., Shishov D., Peselis A., Shamovsky I., Pani B., Lechpammer M., Vasilyev N., Shatalina E., Rebatchouk D., Mironov A., Fedichev P., Serganov A., Nudler E., et. al.
Science,
2021
.

Taydakov I.V., Dutova T.Y., Sidorenko E.N., Krasnoselsky S.S.
Chemistry of Heterocyclic Compounds,
2011
.

Sun Q., Collins R., Huang S., Holmberg-Schiavone L., Anand G.S., Tan C., van-den-Berg S., Deng L., Moore P.K., Karlberg T., Sivaraman J.
Journal of Biological Chemistry,
2009
.
![Efficient Homocoupling of Aryl- and Alkenylboronic Acids in the Presence of Low Loadings of [{Pd(μ–OH)Cl(IPr)}2]](/storage/images/resized/xqixcltwJYe6H8Uco2JbAFfIOzt7UNKH0OcPOPzO_small_thumb.webp)
Pietraszuk C., Ostrowska S., Rogalski S., Lorkowski J., Walkowiak J.
Synlett,
2018
.

Rajappa S., Gumaste V.K.
Advances in Heterocyclic Chemistry,
2013
.

Potapov K.V., Novikov R.A., Novikov M.A., Solyev P.N., Tomilov Y.V., Kochetkov S.N., Makarov A.A., Mitkevich V.A.
Molecules,
2023