Keywords
1
4-naphthoquinones
amines
oxidation
radical anions.
reduction
Abstract
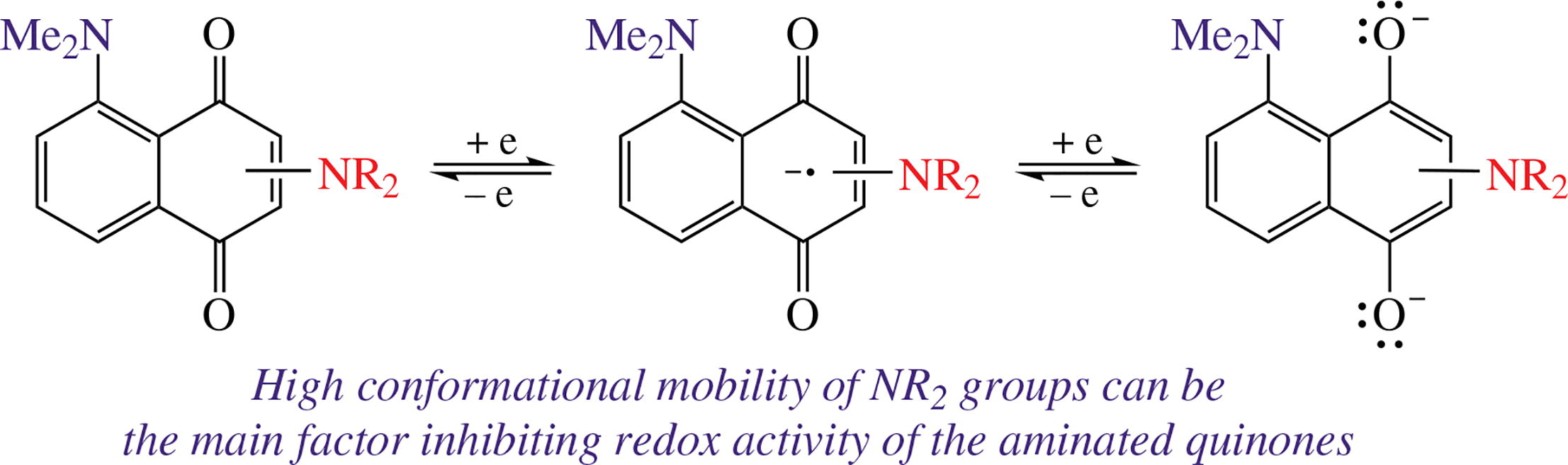
Four representatives of amino derivatives of 1,4-naphtho-quinone were examined by combination of cyclic voltammetry, ESR spectroscopy and quantum chemical calculations. The results obtained help to rationalize why natural quinonoid redox systems do not contain amino groups so common in proteins, nucleobases, numerous signal and other biologically important molecules.
References
.
.
Pozharskii A.F., Soldatenkov A.T., Katritzky A.R.
2011
.

Foster J.P., Weinhold F.
Journal of the American Chemical Society,
1980
.

Alvarez S.
Dalton Transactions,
2013
.

Elgrishi N., Rountree K.J., McCarthy B.D., Rountree E.S., Eisenhart T.T., Dempsey J.L.
Journal of Chemical Education,
2017
.

.

Stone A.J.
Journal of Physical Chemistry A,
2017
.

Karpinska J., Erxleben A., McArdle P.
Crystal Growth and Design,
2013
.
Minkin V.I., Osipov O.A., Zhdanov Y.A.
2012
.

Prabhananda B.S.
Journal of Chemical Physics,
1983
.

Mills J.E., Maryanoff C.A., Cosgrove R.M., Scott L., McComsey D.F.
Organic Preparations and Procedures International,
1984
.

Unden G., Bongaerts J.
Biochimica et Biophysica Acta - Bioenergetics,
1997
.

Pedersen J.A.
Spectrochimica Acta - Part A: Molecular and Biomolecular Spectroscopy,
2002
.
Vlasenko M.P., Pozharskii A.F., Demidov O.P., Ozeryanskii V.A., Borodkin G.S.
Mendeleev Communications,
2023
.

Pozharskii Alexander F., Dyablo Olga V., Ozeryanskii Valery A., Pogosova Olga G.
Russian Chemical Reviews,
2022