Keywords
alkynes
arylboronic acids
carbopalladation
catalysis
dienes.
palladium
transmetalation
Abstract
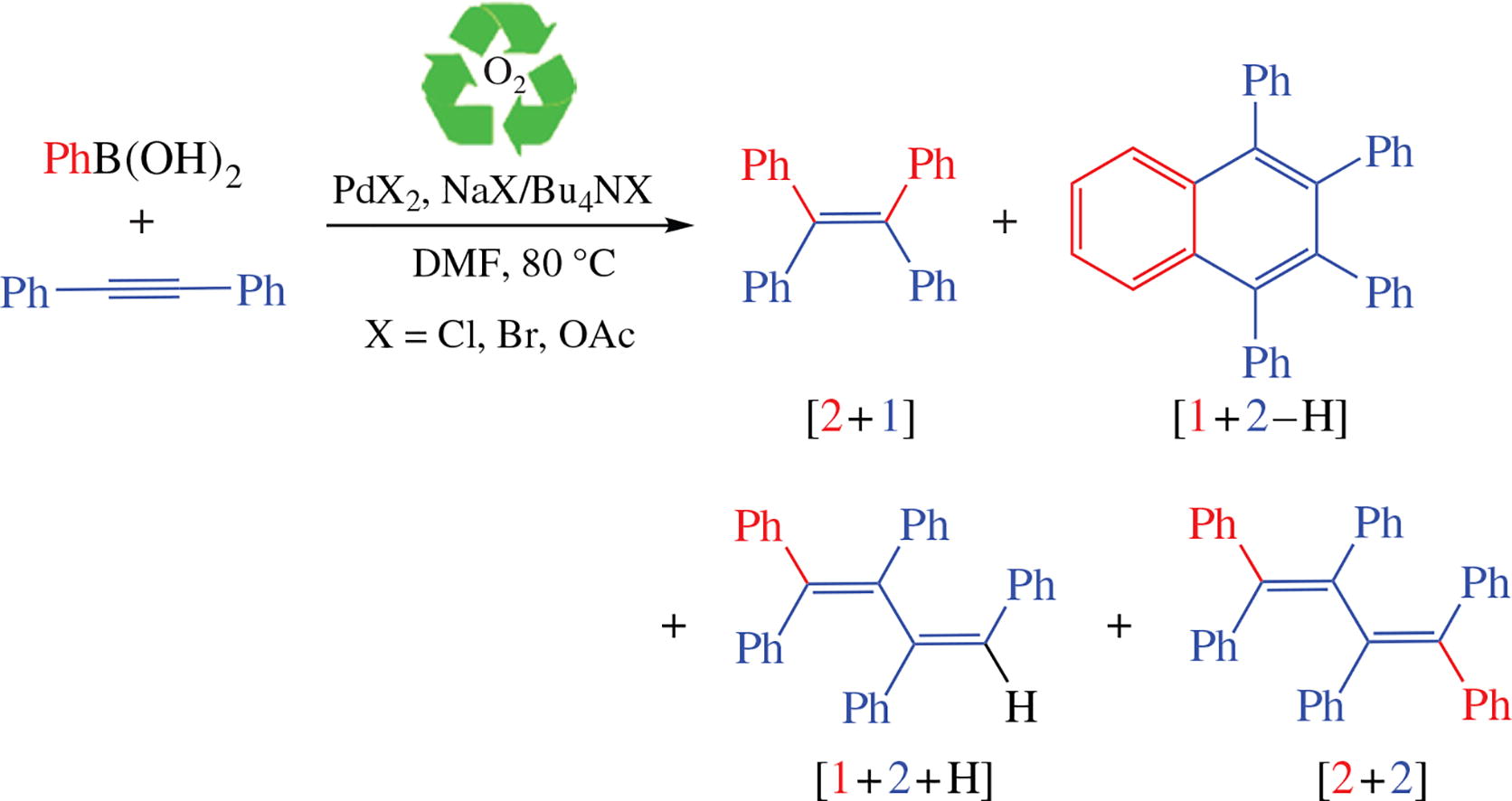
‘Ligand-free’ Pd-catalyzed reaction between arylboronic acid and diphenylacetylene affords a set of polyphenylated olefins and 1,2,3,4-tetraphenylnaphthalene whose yields are dependent on counteranion of PdII salt and additive nature. Tetraphenylethylene and hexaphenylbuta-1,3-diene are likely formed in tandem arylation/cross-coupling reaction with the participation of hydroxo/alkoxo alkenyl Pd species, whereas 1,2,3,4-tetraphenylnaphthalene formation probably proceeds through tandem arylation/C-H activation by halide-containing alkenyl Pd complexes.
References
.

Schmidt A., Al Halaiqa A., Smirnov V.
Synlett,
2006
.

Carrow B.P., Hartwig J.F.
Journal of the American Chemical Society,
2011
.

Schmidt A.F., Kurokhtina A.A., Larina E.V.
Russian Journal of General Chemistry,
2011
.

Amatore C., Jutand A., Le Duc G.
Chemistry - A European Journal,
2011
.

Thomas A.A., Denmark S.E.
Science,
2016
.

de Vries J.G.
Dalton Transactions,
2006
.

Deeth R.J., Smith A., Hii K.K., Brown J.M.
Tetrahedron Letters,
1998
.

Kostyukovich A.Y., Burykina J.V., Eremin D.B., Ananikov V.P.
Inorganic Chemistry,
2021
.

Polynski M.V., Ananikov V.P.
ACS Catalysis,
2019
.

Wu Y., Huang K., Shin C., Wu T.
Chemistry - A European Journal,
2008
.

Kawasaki S., Satoh T., Miura M., Nomura M.
Journal of Organic Chemistry,
2003
.

Zhang X., Larock R.C.
Organic Letters,
2003
.

Zhou C., Larock R.C.
Journal of Organic Chemistry,
2006
.

Fukutani T., Hirano K., Satoh T., Miura M.
Journal of Organic Chemistry,
2011
.

Jafarpour F., Hazrati H., Nouraldinmousa S.
Organic Letters,
2013
.

Bej A., Chakraborty A., Sarkar A.
RSC Advances,
2013
.

Satoh T., Ogino S., Miura M., Nomura M.
Angewandte Chemie - International Edition,
2004
.

Horiguchi H., Tsurugi H., Satoh T., Miura M.
Advanced Synthesis and Catalysis,
2008
.

Xu X., Chen J., Gao W., Wu H., Ding J., Su W.
Tetrahedron,
2010
.
Zhang J., Chen Y., Luo X., Wen Z.
Mendeleev Communications,
2022
.
Xu M., Zhang K., Zhang J.
Mendeleev Communications,
2022
.

Cui Y., Wang R., Yang C., Wang A., Jing Y., Zhang S.
Russian Journal of General Chemistry,
2022