Keywords
1
2
3-triazole
7-azaspiro[4.6]undec-9-ene
8-azaspiro[5.6]dodec-10-ene
azepane
azepine
click chemistry.
metathesis reaction
spirocycle
stereoselective synthesis
Abstract
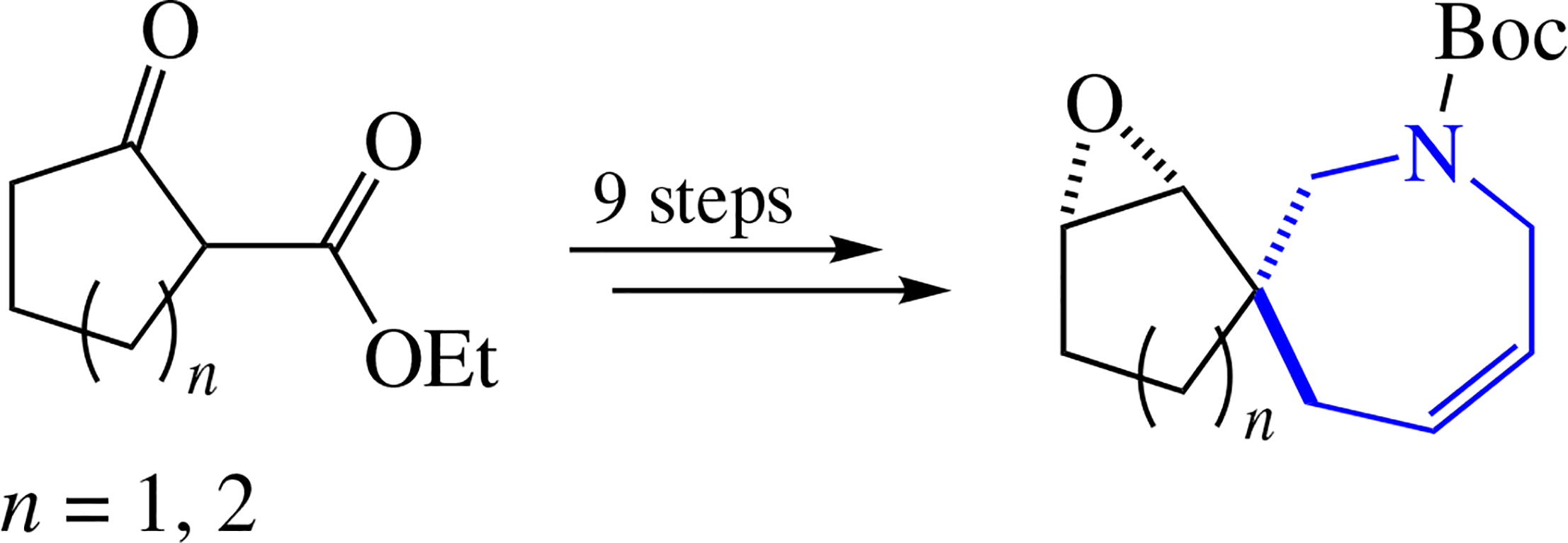
A simple and effective stereoselective synthesis of unique azaspirocyclic 2,3,4,7-tetrahydro-1H-azepine derivatives, namely, 7-azaspiro[4.6]undec-9-ene and 8-azaspiro[5.6]- dodec-10-ene, from simple commercially available reagents was accomplished. Their key 1,2-epoxy derivatives as individual diastereomers were obtained in nine steps with 31-32% overall yields. The possibility of synthesizing a library of small spiroheterocyclic molecules was exemplified by the N-Boc-protected (1RS,2SR,6RS)-1,2-epoxy-8-aza- spiro[5.6]dodec-10-ene scaffold through simple chemical modifications of its epoxide function.
References
.

Zheng Y., Tice C.M., Singh S.B.
Bioorganic and Medicinal Chemistry Letters,
2014
.

Zheng Y., Tice C.M.
Expert Opinion on Drug Discovery,
2016
.

Lovering F., Bikker J., Humblet C.
Journal of Medicinal Chemistry,
2009
.

Marson C.M.
Chemical Society Reviews,
2011
.

.

Aldeghi M., Malhotra S., Selwood D.L., Chan A.W.
Chemical Biology and Drug Design,
2014
.

Walters W.P., Green J., Weiss J.R., Murcko M.A.
Journal of Medicinal Chemistry,
2011
.

Ritchie T.J., Macdonald S.J.
Drug Discovery Today,
2009
.

Sharpless K.B., Michaelson R.C.
Journal of the American Chemical Society,
1973
.

Fürstner A., Langemann K.
Journal of the American Chemical Society,
1997
.

Sirivolu V.R., Vernekar S.K., Ilina T., Myshakina N.S., Parniak M.A., Wang Z.
Journal of Medicinal Chemistry,
2013
.

Kirsch P., Hartman A.M., Hirsch A.K., Empting M.
Molecules,
2019
.

Lipshutz B.H., Ghorai S.
Organic Letters,
2008
.
![Discovery of (R,E)-N-(7-Chloro-1-(1-[4-(dimethylamino)but-2-enoyl]azepan-3-yl)-1H-benzo[d]imidazol-2-yl)-2-methylisonicotinamide (EGF816), a Novel, Potent, and WT Sparing Covalent Inhibitor of Oncogenic (L858R, ex19del) and Resistant (T790M) EGFR Mutants for the Treatment of EGFR Mutant Non-Small-Cell Lung Cancers](/storage/images/resized/iLiQsFqFaSEx6chlGQ5fbAwF6VYU3WWa08hkss0g_small_thumb.webp)
Lelais G., Epple R., Marsilje T.H., Long Y.O., McNeill M., Chen B., Lu W., Anumolu J., Badiger S., Bursulaya B., DiDonato M., Fong R., Juarez J., Li J., Manuia M., et. al.
Journal of Medicinal Chemistry,
2016
.

Reilly S.W., Griffin S., Taylor M., Sahlholm K., Weng C., Xu K., Jacome D.A., Luedtke R.R., Mach R.H.
Journal of Medicinal Chemistry,
2017
.

Drwal M.N., Bret G., Perez C., Jacquemard C., Desaphy J., Kellenberger E.
Journal of Medicinal Chemistry,
2018
.

Li R., Ling D., Tang T., Huang Z., Wang M., Ding Y., Liu T., Wei H., Xu W., Mao F., Zhu J., Li X., Jiang L., Li J.
Journal of Medicinal Chemistry,
2021
.

Iusupov I.R., Lukyanenko E.R., Altieri A., Kurkin A.V.
ChemMedChem,
2022
.

Lindsay V.N., Murphy R.A., Sarpong R.
Chemical Communications,
2017
.

Fu G.C., Nguyen S.T., Grubbs R.H.
Journal of the American Chemical Society,
1993
.

Greger H.
Phytochemistry Reviews,
2019
.

de Lourdes G. Ferreira M., Pinheiro L.C., Santos-Filho O.A., Peçanha M.D., Sacramento C.Q., Machado V., Ferreira V.F., Souza T.M., Boechat N.
Medicinal Chemistry Research,
2013
.
![(1RS,2RS,6RS)-2-(6-Amino-9H-purin-9-yl)-8-azaspiro[5.6]dodec-10-en-1-ol Dihydrochloride](/storage/images/resized/MjH1ITP7lMYGxeqUZfkt2BnVLgjkk413jwBV97XX_small_thumb.webp)
Iusupov I.R., Lyssenko K.A., Altieri A., Kurkin A.V.
MolBank,
2022
.

Koide K., Bunnage M.E., Gomez Paloma L., Kanter J.R., Taylor S.S., Brunton L.L., Nicolaou K.C.
Chemistry & Biology,
1995
.

Nadolski G.T., Davidson B.S.
Tetrahedron Letters,
2001
.

Pandey G., Prasanna Kumara C., Kumar Burugu S., Puranik V.G.
European Journal of Organic Chemistry,
2011
.
![1,3-Di-n-butylimidazolium tribromide [BBim]Br3: An efficient recyclable catalyst mediated synthesis of N-substituted azepines and their biological evaluation-interaction study with human serum albumin](/storage/images/resized/GDnYOu1UpMMfMMRV6Aqle4H0YLLsraeD9IP9qScG_small_thumb.webp)
Maddili S.K., Chowrasia R., Kannekanti V.K., Gandham H.
Journal of Photochemistry and Photobiology B: Biology,
2018
.

Greger H.
Planta Medica,
2006
.

Schmidt B., Hauke S., Mühlenberg N.
Synthesis,
2014
.

Pande V., Ramos M., Gago F.
Anti-Cancer Agents in Medicinal Chemistry,
2008
.

Marco L., Carreiras M.
Recent Patents on CNS Drug Discovery,
2006
.
Alizadeh A., Bagherinejad A.
Mendeleev Communications,
2023