Keywords
asymmetric organocatalysis
benzyloxycarbonyl protective group
chiral secondary alcohols
diethylzinc.
enantioselective addition
ferrocene
ferrocenylmagnesium bromide
proline
X-ray molecular structure
Abstract
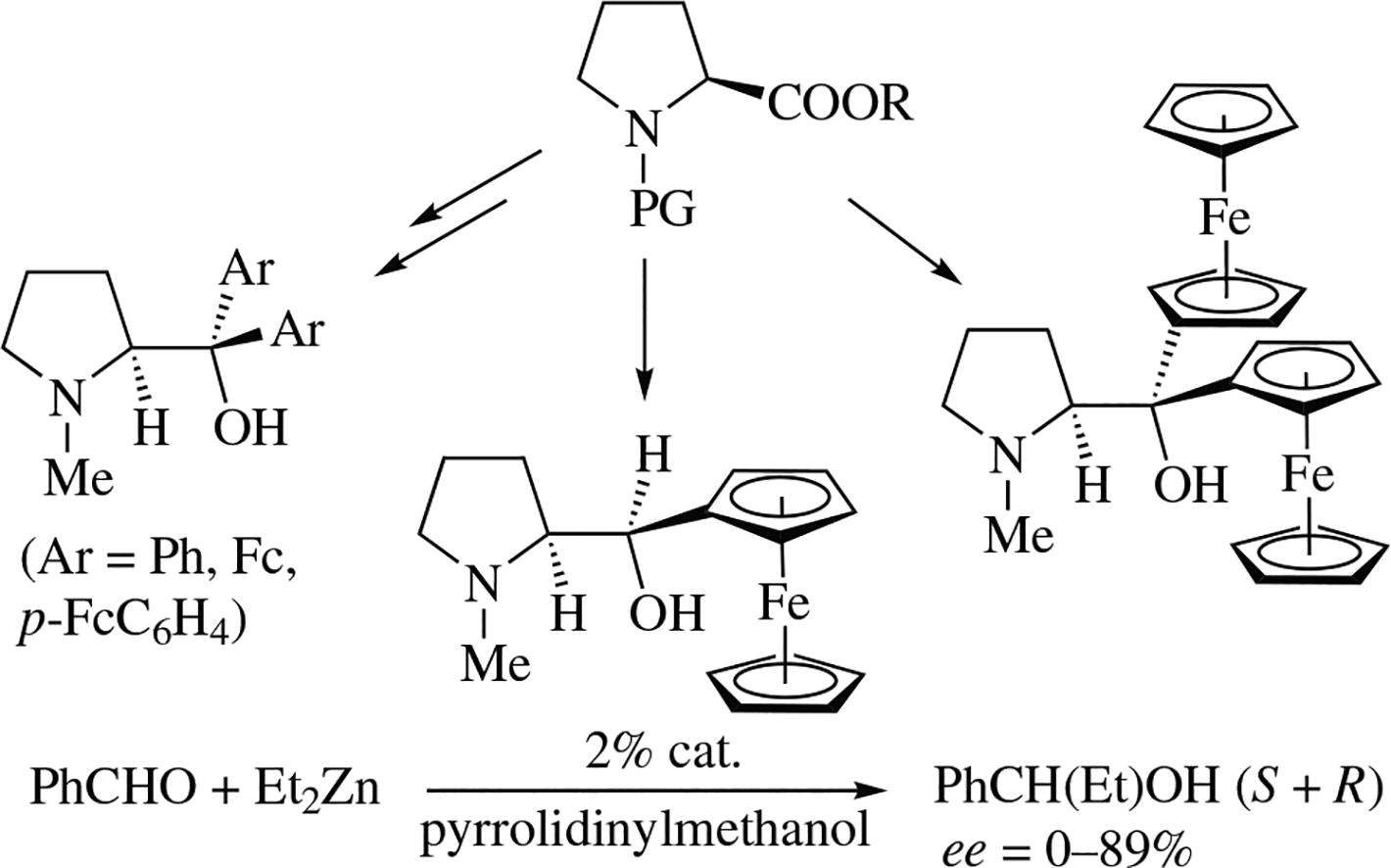
New ferrocenyl-containing diastereomeric secondary alcohols derived from L-proline of (ferrocenyl)(pyrrolidin-2-yl)methanol chemotype were synthesized. Their crystal and molecular structure was explored. The prepared chiral secondary and tertiary alcohols in which hydroxy group is sterically hindered by ferrocene substituents show asymmetric induction effect in reaction between diethylzinc and benzaldehyde.
References
.

Matsev O.V., Beletskaya I.P., Zlotin S.G.
Russian Chemical Reviews,
2011
.

Zlotin S.G., Kucherenko A.S., Beletskaya I.P.
Russian Chemical Reviews,
2009
.

Gruttadauria M., Giacalone F., Noto R.
Chemical Society Reviews,
2008
.

Kucherenko A.S., Siyutkin D.E., Maltsev O.V., Kochetkov S.V., Zlotin S.G.
Russian Chemical Bulletin,
2012
.

List B., Pojarliev P., Biller W.T., Martin H.J.
Journal of the American Chemical Society,
2002
.

France S., Guerin D.J., Miller S.J., Lectka T.
Chemical Reviews,
2003
.

Al-Momani L., Lataifeh A.
Industrial & Engineering Chemistry Research,
2022
.

Soai K., Ookawa A., Kaba T., Ogawa K.
Journal of the American Chemical Society,
1987
.

Kawabata T., Stragies R., Fukaya T., Fuji K.
Chirality,
2002
.
Kochetkov S.V., Kucherenko A.S., Zlotin S.G.
Mendeleev Communications,
2015
.

Bonsignore M., Benaglia M., Raimondi L., Orlandi M., Celentano G.
Beilstein Journal of Organic Chemistry,
2013
.

Soai K., Niwa S.
Chemical Reviews,
1992
.

Panday S.K.
Tetrahedron Asymmetry,
2011
.

Takeuchi Y., Yamada A., Suzuki T., Koizumi T.
Tetrahedron,
1996
.
Burlutsky R.O., Yudina E.S., Churakov A.V., Lemenovskiy D.A., Dyadchenko V.P.
Mendeleev Communications,
2024