Keywords
3-c]quinolin-4(5H)-ones.
arylation
C–H activation
furo[2
N-heterocyclic carbene ligands
palladium
Abstract
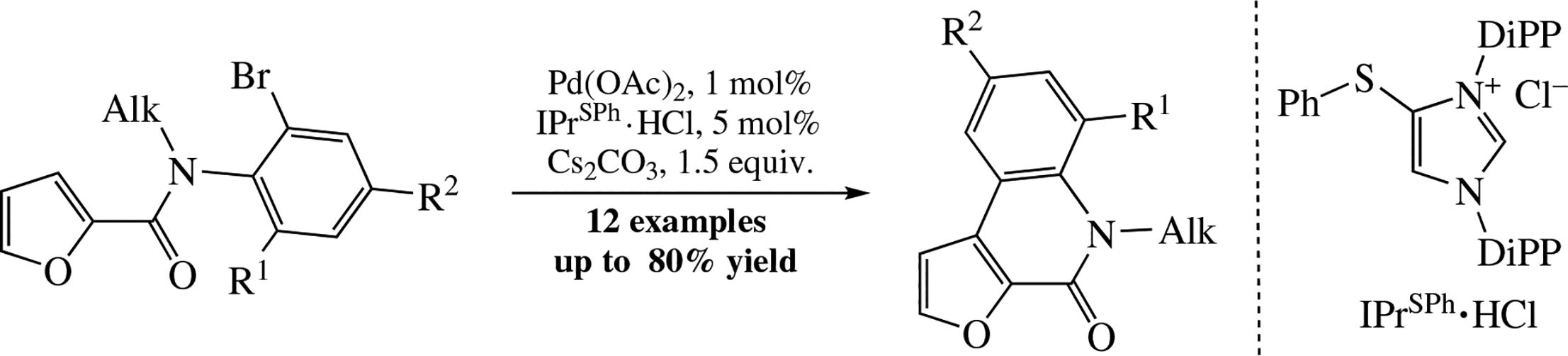
An efficient procedure for the preparation of furo[2,3-c]-quinolin-4(5H)-ones from 2-furoic acid N-(o-bromoaryl)- amides via selective intramolecular C(3)-H arylation of the furan nucleus involves the catalysis by a Pd/NHC system generated in situ from Pd(OAc)2 and readily available IPrSPh HCl proligand. A series of novel furo[2,3-c]quinolin-4(5H)-ones were prepared in 65-80% isolated yields.
References
.

Chernyshev V.M., Denisova E.A., Eremin D.B., Ananikov V.P.
Chemical Science,
2020
.

Karlinskii B.Y., Ananikov V.P.
ChemSusChem,
2020
.

Karlinskii B.Y., Kostyukovich A.Y., Kucherov F.A., Galkin K.I., Kozlov K.S., Ananikov V.P.
ACS Catalysis,
2020
.

Mori A., Curpanen S., Pezzetta C., Perez‐Luna A., Poli G., Oble J.
European Journal of Organic Chemistry,
2022
.
Shepelenko K.E., Nikolaeva K.A., Shevchenko M.A., Tkachenko Y.N., Minyaev M.E., Chernyshev V.M.
Mendeleev Communications,
2022
.

Campeau L., Parisien M., Jean A., Fagnou K.
Journal of the American Chemical Society,
2005
.
![Palladium-catalyzed synthesis of pyrimido[5’,4’:3,4]pyrrolo[1,2-f]phenanthridine-12,14(11H,13H)-diones and related compounds](/storage/images/resized/voXLqlsvTwv5p3iMQ8Dhs95nqB4AXOG7Taj7G4ra_small_thumb.webp)
Shevchenko M.A., Tkachenko Y.N., Astakhov A.V., Khazipov O.V., Tyurin R.V., Pasyukov D.V., Tafeenko V.A., Kravchenko O.A., Chernyshev V.M.
Russian Chemical Bulletin,
2018
.

Michael J.P.
Natural Product Reports,
1997
.

Chernyshev V.M., Khazipov O.V., Shevchenko M.A., Chernenko A.Y., Astakhov A.V., Eremin D.B., Pasyukov D.V., Kashin A.S., Ananikov V.P.
Chemical Science,
2018
.
![Regioselective and Stereoselective Pd-Catalyzed Intramolecular Arylation of Furans: Access to Spirooxindoles and 5H-Furo[2,3-c]quinolin-4-ones.](/storage/images/resized/iLiQsFqFaSEx6chlGQ5fbAwF6VYU3WWa08hkss0g_small_thumb.webp)
Liu J., Peng H., Yang Y., Jiang H., Yin B.
Journal of Organic Chemistry,
2016
.

Pasyukov D.V., Shevchenko M.A., Shepelenko K.E., Khazipov O.V., Burykina J.V., Gordeev E.G., Minyaev M.E., Chernyshev V.M., Ananikov V.P.
Angewandte Chemie - International Edition,
2022
.

Zhao L., Guo X., Liu S., Feng L., Bi Q., Wang Z., Tan N.
Natural Products and Bioprospecting,
2018
.

Khazipov O.V., Shepelenko K.E., Soliev S.B., Nikolaeva K.A., Chernyshev V.M., Ananikov V.P.
ChemCatChem,
2022
.

Yamashita M., Saito Y., Rahim A., Fukuyoshi S., Miyake K., Goto M., Nakagawa-Goto K.
Tetrahedron Letters,
2020
.

Modranka J., Drogosz-Stachowicz J., Pietrzak A., Janecka A., Janecki T.
European Journal of Medicinal Chemistry,
2021
.

Hanawa F., Fokialakis N., Skaltsounis A.
Planta Medica,
2004
.

Zhao W., Wolfender J., Hostettmann K., Xu R., Qin G.
Phytochemistry,
1998
.

Al-Rehaily A.J., Ahmad M.S., Muhammad I., Al-Thukair A.A., Perzanowski H.P.
Phytochemistry,
2003
.
Abe H., Kamimura M., Komatsu Y., Horino Y.
Heterocycles,
2015
.

Pasyukov D., Shevchenko M., Astakhov A.V., Minyaev M.E., Zhang Y., Chernyshev V., Ananikov V.P.
Dalton Transactions,
2023
.

Szewczyk A., Pęczek F.
International Journal of Molecular Sciences,
2023