Keywords
1
2
3
3-benzothiadiazole
5-triyl
absorption spectrum
benzene- 1
cross-coupling
DFT calculations.
fluorescence quantum yield
fluorescence spectrum
oligophenylenes
organic luminophore
Abstract
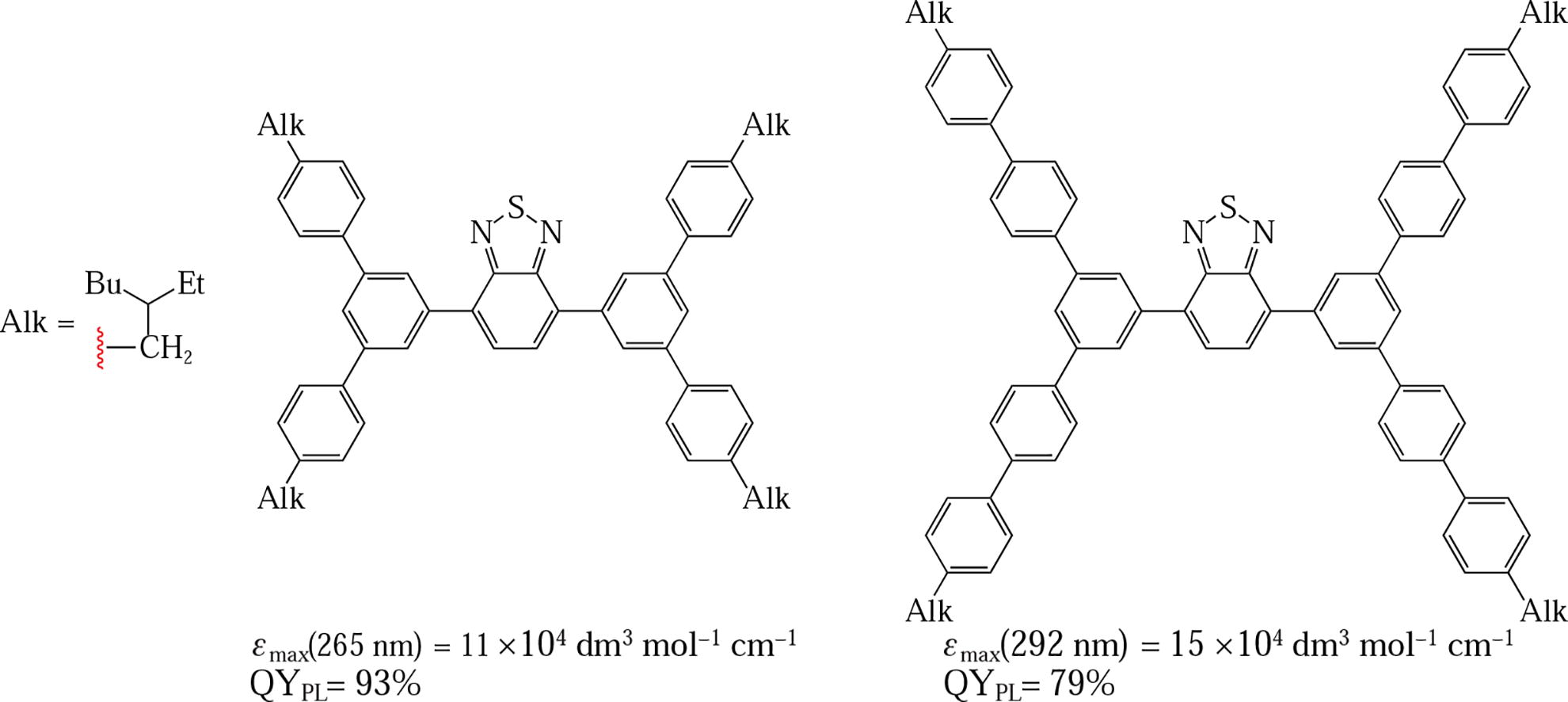
Synthesis and examination of two highly luminescent oligophenylene derivatives of 2,1,3-benzothiadiazole with branching centers based on benzene-1,3,5-triyl units are described. These studies in combination with DFT calculations have shown that the presence of the branching centers causes differences in the absorption characteristics and similarity of the emission properties. High molar extinction coefficients (up to 15 × 104 dm3 mol−1 cm−1) in the medium UV-range and blue-green emission with quantum yields of 79-93% make them promising components of wavelength-shifting materials for optical devices.
References
.

Gudim N.S., Knyazeva E.A., Mikhalchenko L.V., Golovanov I.S., Popov V.V., Obruchnikova N.V., Rakitin O.A.
Molecules,
2021
.

Neese F., Wennmohs F., Becker U., Riplinger C.
Journal of Chemical Physics,
2020
.
Gribanov P.S., Loginov D.A., Lypenko D.A., Dmitriev A.V., Tokarev S.D., Aleksandrov A.E., Tameev A.R., Chernyadyev A.Y., Osipov S.N.
Mendeleev Communications,
2023
.

Mako T.L., Racicot J.M., Levine M.
Chemical Reviews,
2018
.

DaSilveira Neto B.A., Lopes A.S., Ebeling G., Gonçalves R.S., Costa V.E., Quina F.H., Dupont J.
Tetrahedron,
2005
.

Crosby G.A., Demas J.N.
The Journal of Physical Chemistry,
1971
.
![Color Tuning in Benzo[1,2,5]thiadiazole‐Based Small Molecules by Amino Conjugation/Deconjugation: Bright Red‐Light‐Emitting Diodes](/storage/images/resized/bRyGpdm98BkAUYiK1YFNpl5Z7hPu6Gd87gbIeuG3_small_thumb.webp)
Justin Thomas K. ., Lin J. ., Velusamy M., Tao Y.-., Chuen C.-.
Advanced Functional Materials,
2004
.

Lee S., Chang C.
Polymers,
2019
.

Chang Z., Jing L., Wei C., Dong Y., Ye Y., Zhao Y.S., Wang J.
Chemistry - A European Journal,
2015
.

Yamaguchi S., Akiyama S., Tamao K.
Journal of the American Chemical Society,
2000
.
![Photoinduced Electron Transfer in an Anthraquinone–[Ru(bpy)3]2+–Oligotriarylamine–[Ru(bpy)3]2+–Anthraquinone Pentad](/storage/images/resized/iLiQsFqFaSEx6chlGQ5fbAwF6VYU3WWa08hkss0g_small_thumb.webp)
Bonn A.G., Yushchenko O., Vauthey E., Wenger O.S.
Inorganic Chemistry,
2016
.
![Phenolic Bis-styrylbenzo[c]-1,2,5-thiadiazoles as Probes for Fluorescence Microscopy Mapping of Aβ Plaque Heterogeneity](/storage/images/resized/iLiQsFqFaSEx6chlGQ5fbAwF6VYU3WWa08hkss0g_small_thumb.webp)
Zhang J., Konsmo A., Sandberg A., Wu X., Nyström S., Obermüller U., Wegenast-Braun B.M., Konradsson P., Lindgren M., Hammarström P.
Journal of Medicinal Chemistry,
2019
.

Villa M., Ceroni P., Fermi A.
ChemPlusChem,
2022
.

Skorotetcky M.S., Borshchev O.V., Cherkaev G.V., Ponomarenko S.A.
Russian Journal of Organic Chemistry,
2019
.

Goubard F., Dumur F.
RSC Advances,
2015
.

Ponomarenko S.A., Surin N.M., Skorotetcky M.S., Borshchev O.V., Pisarev S.A., Svidchenko E.A., Fedorov Y.V., Molins F., Brixner T.
Journal of Materials Chemistry C,
2019
.

Brady L.E.
Journal of Medicinal Chemistry,
1966
.

Ponomarenko S.A., Surin N.M., Borshchev O.V., Luponosov Y.N., Akimov D.Y., Alexandrov I.S., Burenkov A.A., Kovalenko A.G., Stekhanov V.N., Kleymyuk E.A., Gritsenko O.T., Cherkaev G.V., Kechek'yan A.S., Serenko O.A., Muzafarov A.M., et. al.
Scientific Reports,
2014
.

Skorotetcky M.S., Surin N.M., Svidchenko E.A., Pisarev S.A., Fedorov Y.V., Borshchev O.V., Kuleshov B.S., Shaposhnik P.A., Maloshitskaya O.A., Ponomarenko S.A.
Journal of Physical Chemistry B,
2022
.

Luponosov Y.N., Solodukhin A.N., Balakirev D.O., Surin N.M., Svidchenko E.A., Pisarev S.A., Fedorov Y.V., Ponomarenko S.A.
Dyes and Pigments,
2020
.

Skorotetcky M.S., Krivtsova E.D., Borshchev O.V., Surin N.M., Svidchenko E.A., Fedorov Y.V., Pisarev S.A., Ponomarenko S.A.
Dyes and Pigments,
2018
.
Borshchev O.V., Surin N.M., Skorotetcky M.S., Ponomarenko S.A.
INEOS OPEN,
2019
.
Chukhlantseva A.N., Dmitriev M.V., Maiorova O.A., Shklyaeva E.V., Abashev G.G.
Mendeleev Communications,
2022