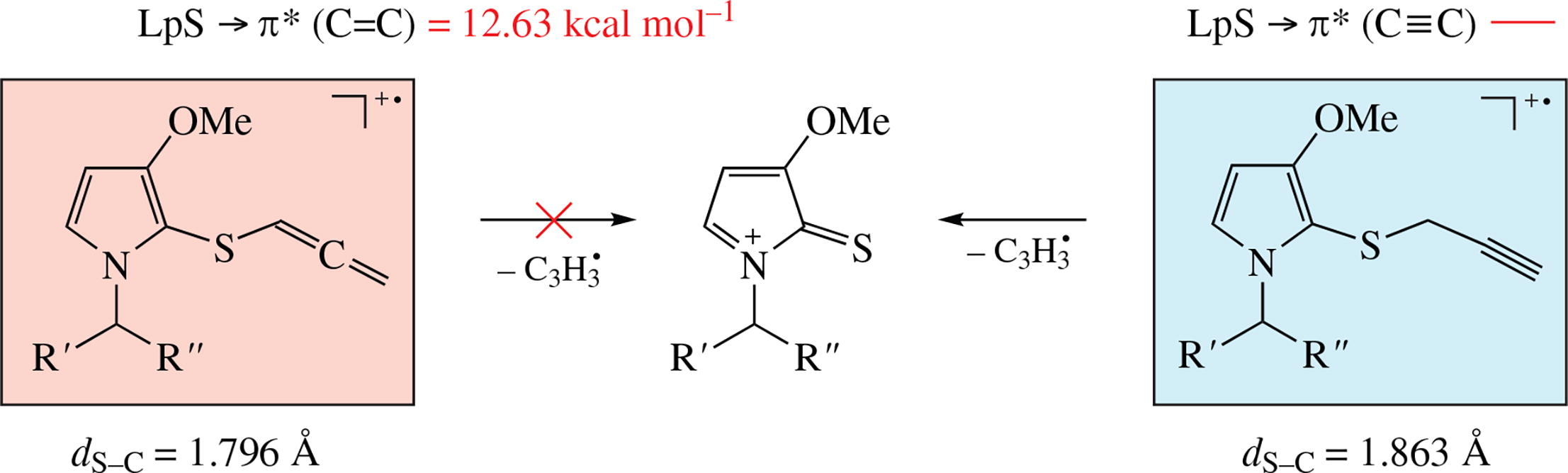
Abstract
The anomalous primary fragmentation (no C–S bond cleavage) in the electron-ionization (70 eV) mass spectrum of 1-isopropyl-3-methoxy-2-(propa-1,2-dien-1-ylthio)-1H- pyrrole is considered in terms of intramolecular electronic effect. The calculation of the geometry (B3PW91/6-311G**) and the study of electron density delocalization (NBO) revealed the effect of electron density transfer LpS → π* (from the lone pair of sulfur atom to the π-system of allene double bond) on primary fragmentation pathways.
References
1.

Reed A.E., Curtiss L.A., Weinhold F.
Chemical Reviews,
1988
2.

Unverferth K., Engel J., Höfgen N., Rostock A., Günther R., Lankau H., Menzer M., Rolfs A., Liebscher J., Müller B., Hofmann H.
Journal of Medicinal Chemistry,
1998
3.

Klyba L.V., Tarasova O.A., Nedolya N.A.
Russian Journal of Organic Chemistry,
2016
4.
![Mass spectra of new heterocycles: XI. Fragmentation of 5-(methylsulfanyl)-1-[2-(vinyloxy)ethyl]-1H-pyrrol-2-amines under electron impact and chemical ionization](/storage/images/resized/oZgeErrVFhuDksyqFURLvYS1wtVSBWczh001igGo_small_thumb.webp)
Klyba L.V., Nedolya N.A., Tarasova O.A., Sanzheeva E.R.
Russian Journal of Organic Chemistry,
2013
5.

Klyba L.V., Bochkarev V.N., Brandsma L., Nedolya N.A., Trofimov B.A.
Russian Chemical Bulletin,
2001
6.

Joshi S., More U., Kulkarni V., Aminabhavi T.
Current Organic Chemistry,
2013