Keywords
C–H annulation
cross-coupling
homogeneous catalysis.
isocoumarins
luminescence
Abstract
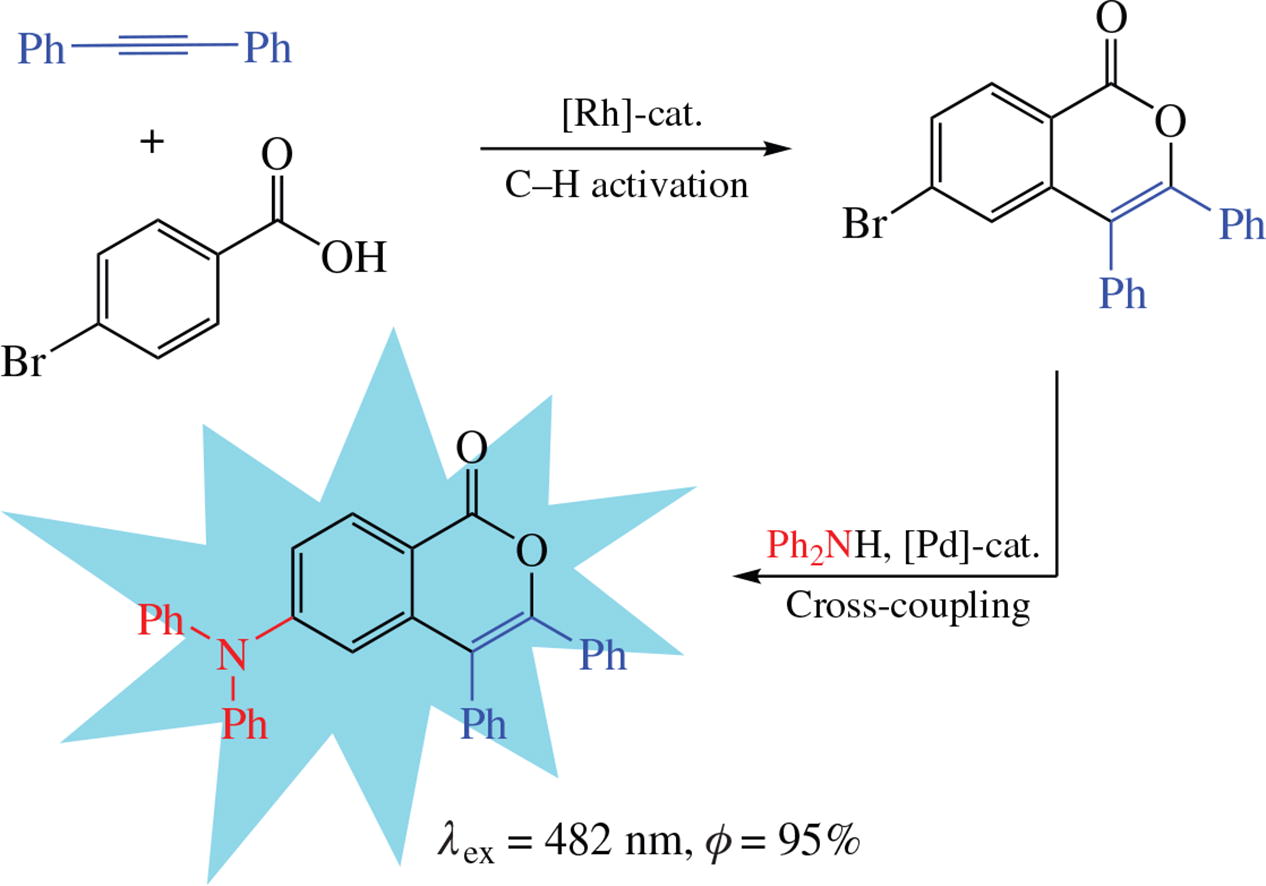
6-Substituted isocoumarins with aryl, alkynyl, and diphenyl-amino substituents were synthesized from 4-bromobenzoic acid by annulation with tolane via rhodium-catalyzed C-H activation followed by palladium-catalyzed cross-coupling reactions. The compounds obtained exhibit luminescence emission in the violet-blue region (370-480 nm) with quantum yields up to 95% (for the diphenylamino derivative). Aggregation of biphenyl-substituted isocoumarin leads to a strong bathochromic shift (by 80 nm) of emission as a result of intermolecular π-π stacking interactions.
References
.

Sheldrick G.M.
Acta Crystallographica Section A: Foundations and Advances,
2015
.

Dolomanov O.V., Bourhis L.J., Gildea R.J., Howard J.A., Puschmann H.
Journal of Applied Crystallography,
2009
.

Sheldrick G.M.
Acta Crystallographica Section A Foundations of Crystallography,
2007
.
Gribanov P.S., Lypenko D.A., Dmitriev A.V., Pozin S.I., Topchiy M.A., Asachenko A.F., Loginov D.A., Osipov S.N.
Mendeleev Communications,
2021
.

Satoh T., Miura M.
Chemistry - A European Journal,
2010
.

Mei J., Leung N.L., Kwok R.T., Lam J.W., Tang B.Z.
Chemical Reviews,
2015
.

Cao D., Liu Z., Verwilst P., Koo S., Jangjili P., Kim J.S., Lin W.
Chemical Reviews,
2019
.

Tasior M., Kim D., Singha S., Krzeszewski M., Ahn K.H., Gryko D.T.
Journal of Materials Chemistry C,
2015
.

Molotkov A.P., Arsenov M.A., Kapustin D.A., Muratov D.V., Shepel' N.E., Fedorov Y.V., Smol'yakov A.F., Knyazeva E.I., Lypenko D.A., Dmitriev A.V., Aleksandrov A.E., Maltsev E.I., Loginov D.A.
ChemPlusChem,
2020
.

Pichette Drapeau M., Gooßen L.J.
Chemistry - A European Journal,
2016
.

Loginov D.A., Konoplev V.E.
Journal of Organometallic Chemistry,
2018
.

Warratz S., Kornhaaß C., Cajaraville A., Niepötter B., Stalke D., Ackermann L.
Angewandte Chemie - International Edition,
2015
.

Han T., Deng H., Yu C.Y., Gui C., Song Z., Kwok R.T., Lam J.W., Tang B.Z.
Polymer Chemistry,
2016
.

Saikia P., Gogoi S.
Advanced Synthesis and Catalysis,
2018
.

Wu J., Qian B., Liu Y., Shang Y.
ChemistrySelect,
2020
.

Ma S., Du S., Pan G., Dai S., Xu B., Tian W.
Aggregate,
2021
.

Gribanov P.S., Vorobyeva D.V., Tokarev S.D., Petropavlovskikh D.A., Loginov D.A., Nefedov S.E., Dolgushin F.M., Osipov S.N.
European Journal of Organic Chemistry,
2022
.

Sun X., Liu T., Sun J., Wang X.
RSC Advances,
2020
.

Arsenov M.A., Fedorov Y.V., Muratov D.V., Nelyubina Y.V., Loginov D.A.
Dyes and Pigments,
2022
.
Arsenov M.A., Loginov D.A.
INEOS OPEN,
2021
.

Qian S., Zhang H., Lan J., Bin Z.
Organic Electronics,
2020
.

Pirovano V., Marchetti M., Carbonaro J., Brambilla E., Rossi E., Ronda L., Abbiati G.
Dyes and Pigments,
2020
.

Kinder M.A., Kopf J., Margaretha P.
Tetrahedron,
2000
.

Vidyakina A., Shtyrov A.A., Ryazantsev M., Khlebnikov A.F., Kolesnikov I.E., Sharoyko V.V., Spiridonova D.V., Balova I.A., Bräse S., Danilkina N.A.
Chemistry - A European Journal,
2023
.

Arsenov M.A., Muratov D.V., Nelyubina Y.V., Loginov D.A.
Journal of Organic Chemistry,
2023
.

Komarova A.A., Perekalin D.S.
Organometallics,
2023
.
Gribanov P.S., Loginov D.A., Lypenko D.A., Dmitriev A.V., Tokarev S.D., Aleksandrov A.E., Tameev A.R., Chernyadyev A.Y., Osipov S.N.
Mendeleev Communications,
2023