Keywords
asymmetric organocatalysis
chiral tertiary alcohols
ferrocene
ferrocenylmagnesium bromide
proline
tert-butoxycarbonyl protective group
X-ray molecular structure.
Abstract
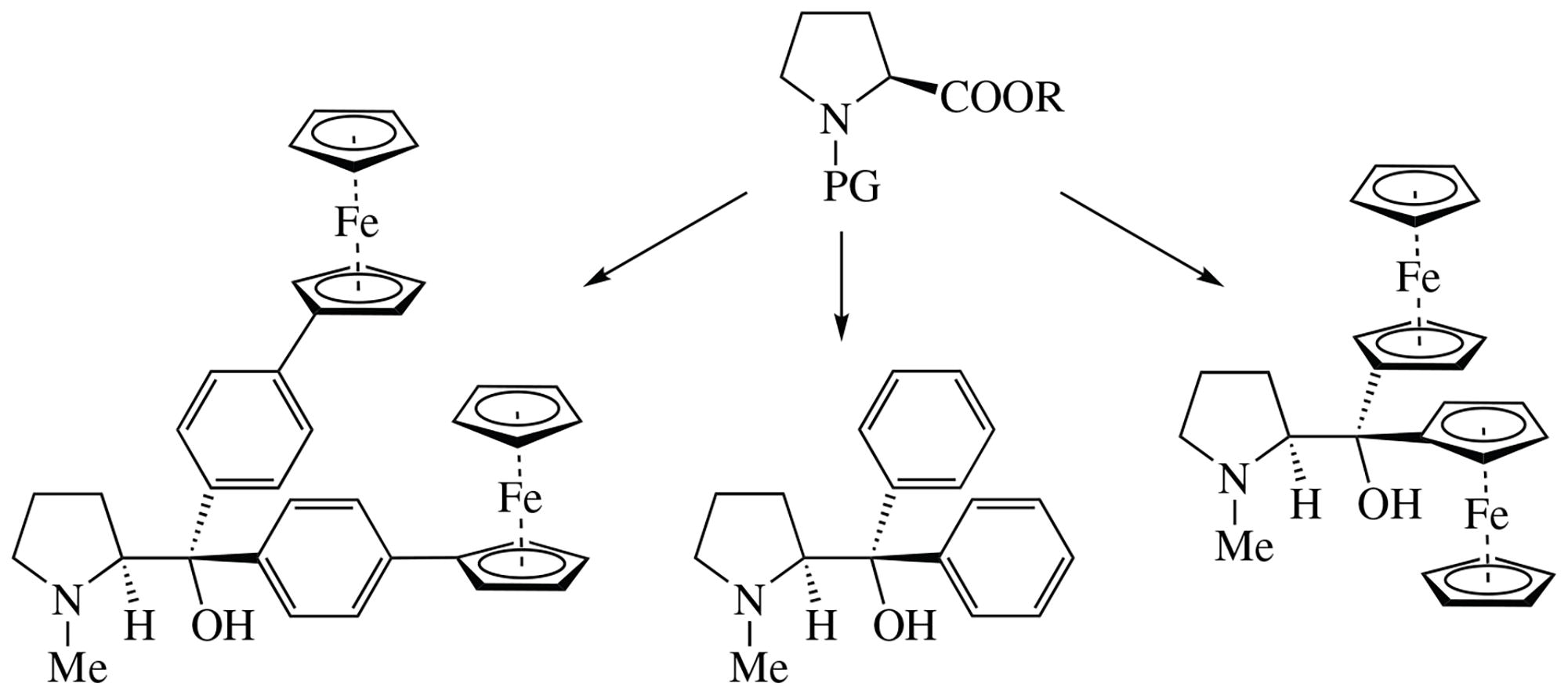
Syntheses of new ferrocenyl-containing tertiary alcohols derived from l-proline were developed. In these alcohols,two ferrocenyl groups are bonded at prolinol moiety directly or through p-phenylene spacer. Specific features for the ferrocene Grignard reagents were discovered.
References
.

Matsev O.V., Beletskaya I.P., Zlotin S.G.
Russian Chemical Reviews,
2011
.

Zlotin S.G., Kucherenko A.S., Beletskaya I.P.
Russian Chemical Reviews,
2009
.

Gruttadauria M., Giacalone F., Noto R.
Chemical Society Reviews,
2008
.

Little W.F., Clark A.K., Benner G.S., Noe C.
Journal of Organic Chemistry,
1964
.

Kucherenko A.S., Siyutkin D.E., Maltsev O.V., Kochetkov S.V., Zlotin S.G.
Russian Chemical Bulletin,
2012
.

List B., Pojarliev P., Biller W.T., Martin H.J.
Journal of the American Chemical Society,
2002
.

Takusagawa F., Koetzle T.F.
Acta Crystallographica Section B,
1979
.

Krištofíková D., Modrocká V., Mečiarová M., Šebesta R.
ChemSusChem,
2020
.

France S., Guerin D.J., Miller S.J., Lectka T.
Chemical Reviews,
2003
.

Al-Momani L., Lataifeh A.
Industrial & Engineering Chemistry Research,
2022
.

Soai K., Ookawa A., Kaba T., Ogawa K.
Journal of the American Chemical Society,
1987
.

Lukasser J., Angleitner H., Schottenberger H., Kopacka H., Schweiger M., Bildstein B., Ongania K., Wurst K.
Organometallics,
1995
.

Kawabata T., Stragies R., Fukaya T., Fuji K.
Chirality,
2002
.
Kochetkov S.V., Kucherenko A.S., Zlotin S.G.
Mendeleev Communications,
2015
.

Bonsignore M., Benaglia M., Raimondi L., Orlandi M., Celentano G.
Beilstein Journal of Organic Chemistry,
2013
.

Axford L.C., Holden K.E., Hasse K., Banwell M.G., Steglich W., Wagler J., Willis A.C.
Australian Journal of Chemistry,
2008