Keywords
5-fluoro-L-DOPA
5-fluoro-L-tyrosine
Amino acids.
Chemoenzymatic method
enantioselective synthesis
organofluorine compounds
Tyrosin phenol lyase
Abstract
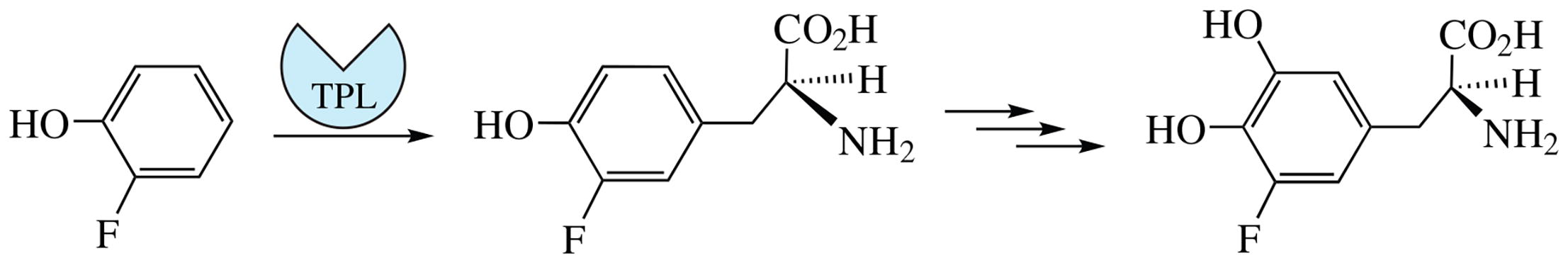
The title compound, 5-fluoro-3,4-dihydroxy-L-phenylalanine, was prepared in four steps, with the key step having been the enantiospecific production of 5-fluoro-L-tyrosine by the chemoenzymatic reaction between 2-fluorophenol, potassium pyruvate and ammonia promoted by the live culture of Citrobacter freundii cells. 5-Fluoro-L-tyrosine was hydroxylated by sequential nitration, reduction and diazotization followed by hydrolysis.
References
.
Galkin K.I.
Mendeleev Communications,
2022
.

Politanskaya L.V., Selivanova G.A., Panteleeva E.V., Tretyakov E.V., Platonov V.E., Nikul’shin P.V., Vinogradov A.S., Zonov Y.V., Karpov V.M., Mezhenkova T.V., Vasilyev A.V., Koldobskii A.B., Shilova O.S., Morozova S.M., Burgart Y.V., et. al.
Russian Chemical Reviews,
2019
.

Gillis E.P., Eastman K.J., Hill M.D., Donnelly D.J., Meanwell N.A.
Journal of Medicinal Chemistry,
2015
.
Grigorenko B.L., Kots E.D., Krylov A.I., Nemukhin A.V.
Mendeleev Communications,
2019
.

Sviridova L.A., Golubeva G.A., Tavtorkin A.N., Kochetkov K.A.
Amino Acids,
2012
.
Alferov K.V., Zhukov Y.N., Khurs E.N., Khomutov R.M.
Mendeleev Communications,
2003
.

Wu S., Snajdrova R., Moore J.C., Baldenius K., Bornscheuer U.T.
Angewandte Chemie - International Edition,
2020
.
![6-[18F]Fluoro-L-DOPA: A Well-Established Neurotracer with Expanding Application Spectrum and Strongly Improved Radiosyntheses](/storage/images/resized/hqfzhQAjTGlNSRs6yzFNITgjSMm9Jr2QuotJHIvE_small_thumb.webp)
Pretze M., Wängler C., Wängler B.
BioMed Research International,
2014
.
![Enzymatic synthesis of no-carrier-added 6-[18F]fluoro-l-dopa with β-tyrosinase](/storage/images/resized/GDnYOu1UpMMfMMRV6Aqle4H0YLLsraeD9IP9qScG_small_thumb.webp)
Kaneko S., Ishiwata K., Hatano K., Omura H., Ito K., Senda M.
Applied Radiation and Isotopes,
1999
.

Moschner J., Stulberg V., Fernandes R., Huhmann S., Leppkes J., Koksch B.
Chemical Reviews,
2019
.
Mostovaya O.A., Valiullina Y.A., Chan C.T., Potrekeeva O.S., Padnya P.L., Zuev Y.F., Stoikov I.I.
Mendeleev Communications,
2019
.

Patti A., Sanfilippo C.
International Journal of Molecular Sciences,
2022
.

Hedges J.B., Ryan K.S.
Chemical Reviews,
2019
.

Dwivedee B.P., Soni S., Sharma M., Bhaumik J., Laha J.K., Banerjee U.C.
ChemistrySelect,
2018
.

Sengupta S., Chandrasekaran S.
Organic and Biomolecular Chemistry,
2019
.

Niemann C., Rapport M.M.
Journal of the American Chemical Society,
1946
.

Lamberth C.
Amino Acids,
2016
.

Do Q., Nguyen G.T., Phillips R.S.
Amino Acids,
2016
.

Markvicheva E.A., Kuptsova S.V., Mareeva T.Y., Vikhrov A.A., Dugina T.N., Strukova S.M., Belokon Y.N., Kochetkov K.A., Baranova E.N., Zubov V.P., Poncelet D., Parmar V.S., Kumar R., Rumsh L.D.
Applied Biochemistry and Biotechnology,
2000
.

Plieva F.M., Kochetkov K.A., Singh I., Parmar V.S., Belokon' Y.N., Lozinsky V.I.
Biotechnology Letters,
2000
.

Phillips R.S., Ravichandran K., Von Tersch R.L.
Enzyme and Microbial Technology,
1989
.

NAGASAWA T., UTAGAWA T., GOTO J., KIM C., TANI Y., KUMAGAI H., YAMADA H.
FEBS Journal,
2005
.
Obydennov D.L., El-Tantawy A.I., Sosnovskikh V.Y.
Mendeleev Communications,
2019
.
Kochetkov K.A., Galkina M.A., Galkin O.M.
Mendeleev Communications,
2010
.

Koyanagi T., Katayama T., Suzuki H., Nakazawa H., Yokozeki K., Kumagai H.
Journal of Biotechnology,
2005
.

Ates S., Cortenlioglu E., Bayraktar E., Mehmetoglu U.
Enzyme and Microbial Technology,
2007
.
![The effect of aromatic fluorine substitution in l-DOPA on the in vivo behaviour of []2-, []5- and []6-fluoro-l-DOPA in the human brain](/storage/images/resized/GDnYOu1UpMMfMMRV6Aqle4H0YLLsraeD9IP9qScG_small_thumb.webp)
Chirakal R., Vasdev N., Asselin M., Schrobilgen G.J., Nahmias C.
Journal of Fluorine Chemistry,
2002
.

Deng W., Wong K.A., Kirk K.
Tetrahedron Asymmetry,
2002
.
![Efficient synthesis of the 18F-labelled 3-O-methyl-6-[18F]fluoro-L-DOPA](/storage/images/resized/GDnYOu1UpMMfMMRV6Aqle4H0YLLsraeD9IP9qScG_small_thumb.webp)
Füchtner F., Steinbach J.
Applied Radiation and Isotopes,
2003
.

Yufryakov V.S., Tsvetikova M.A., Bystrova N.A., Kochetkov K.A.
Russian Chemical Bulletin,
2023
.

Pałka K., Podsadni K., Pająk M.
Journal of Labelled Compounds and Radiopharmaceuticals,
2023