Keywords
1
2-dihydro-3H-pyrrole-3
3
4
4-c]pyridine-3a
4-tetracyanoalkanones
4-tricarbonitriles
5-dihydro-1H-pyrrolo[3
7a-dicarbonitriles.
formaldehyde dimethylhydrazone
N
N-dimethylhydrazine
tetracyanoethylene
Thorpe–Ziegler type cyclization
Abstract
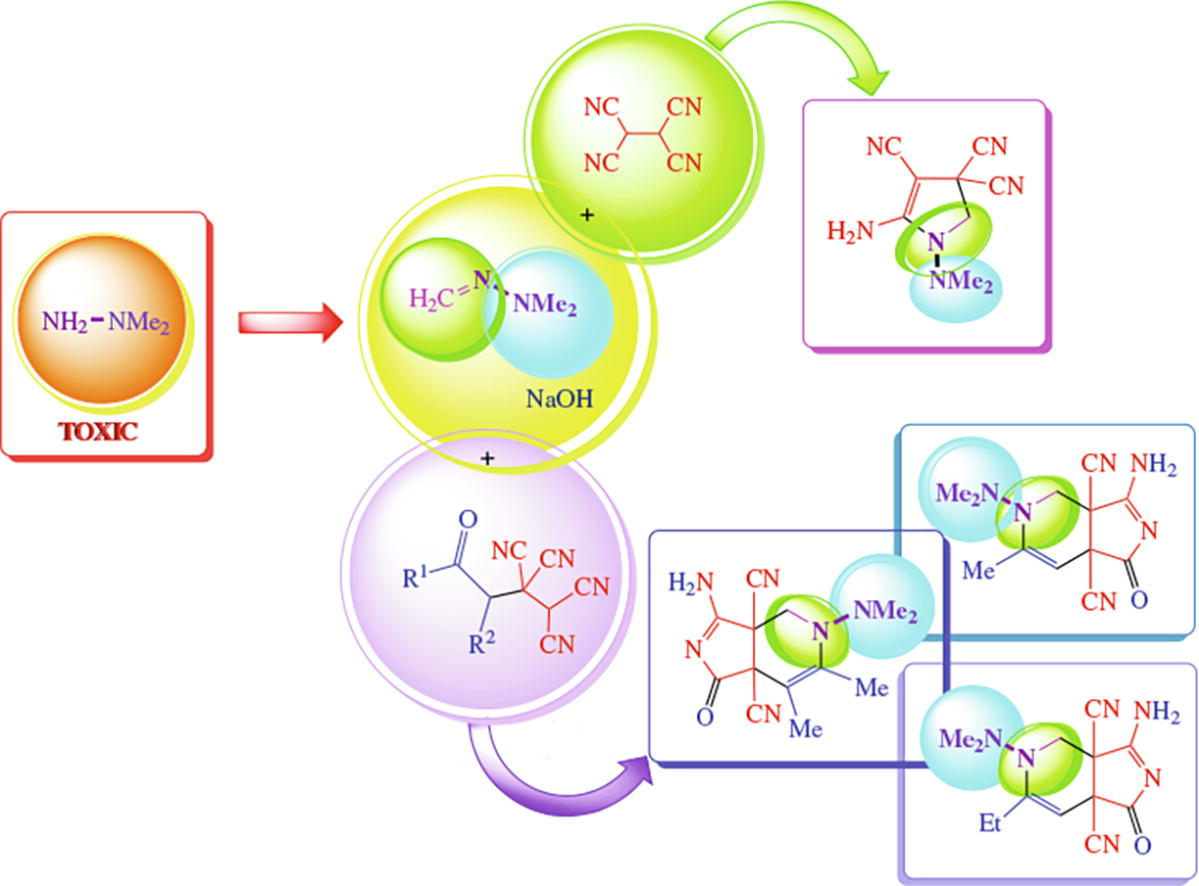
To utilize N,N-dimethylhydrazine, the corresponding +formaldehyde hydrazone was reacted with 1,1,2,2-tetra-cyanoethane or 3,3,4,4-tetracyanoalkanones. The first process yielded 5-amino-1-dimethylamino-1,2-dihydro-3H-pyrrole-3,3,4-tricarbonitrile while the second afforded pyrrolo[3,4-c]quinolone multifunctional derivatives. These resultant products hold promise in molecular design and pharmaceutical chemistry.
References
.
![Domino synthesis of 3-amino-8-hydroxy-1,6-dioxo-2,7-diazaspiro[4.4]non-3-ene-4-carbonitriles](/storage/images/resized/GDnYOu1UpMMfMMRV6Aqle4H0YLLsraeD9IP9qScG_small_thumb.webp)
Fedoseev S.V., Ershov O.V., Belikov M.Y., Lipin K.V., Bardasov I.N., Nasakin O.E., Tafeenko V.A.
Tetrahedron Letters,
2013
.

Belikov M.Y., Ershov O.V., Eremkin A.V., Kayukov Y.S., Nasakin O.E.
Russian Journal of Organic Chemistry,
2010
.

Kamitori Y., Hojo M., Masuda R., Fujitani T., Ohara S., Yokoyama T.
Journal of Organic Chemistry,
1988
.

Singh S.B., Goetz M.A., Jones E.T., Bills G.F., Giacobbe R.A., Herranz L., Stevens-Miles S., Williams D.L.
Journal of Organic Chemistry,
1995
.

Micheli F., Pasquarello A., Tedesco G., Hamprecht D., Bonanomi G., Checchia A., Jaxa-Chamiec A., Damiani F., Davalli S., Donati D., Gallotti C., Petrone M., Rinaldi M., Riley G., Terreni S., et. al.
Bioorganic and Medicinal Chemistry Letters,
2006
.

Butora G., Morriello G.J., Kothandaraman S., Guiadeen D., Pasternak A., Parsons W.H., MacCoss M., Vicario P.P., Cascieri M.A., Yang L.
Bioorganic and Medicinal Chemistry Letters,
2006
.

Fardis M., Jin H., Jabri S., Cai R.Z., Mish M., Tsiang M., Kim C.U.
Bioorganic and Medicinal Chemistry Letters,
2006
.

Toja E., Gorini C., Zirotti C., Barzaghi F., Galliani G.
European Journal of Medicinal Chemistry,
1991