Keywords
anesthetic substances
aromatic amines.
hydrazines
pyrazolecarbonitriles
tetracyanoethylene
Abstract
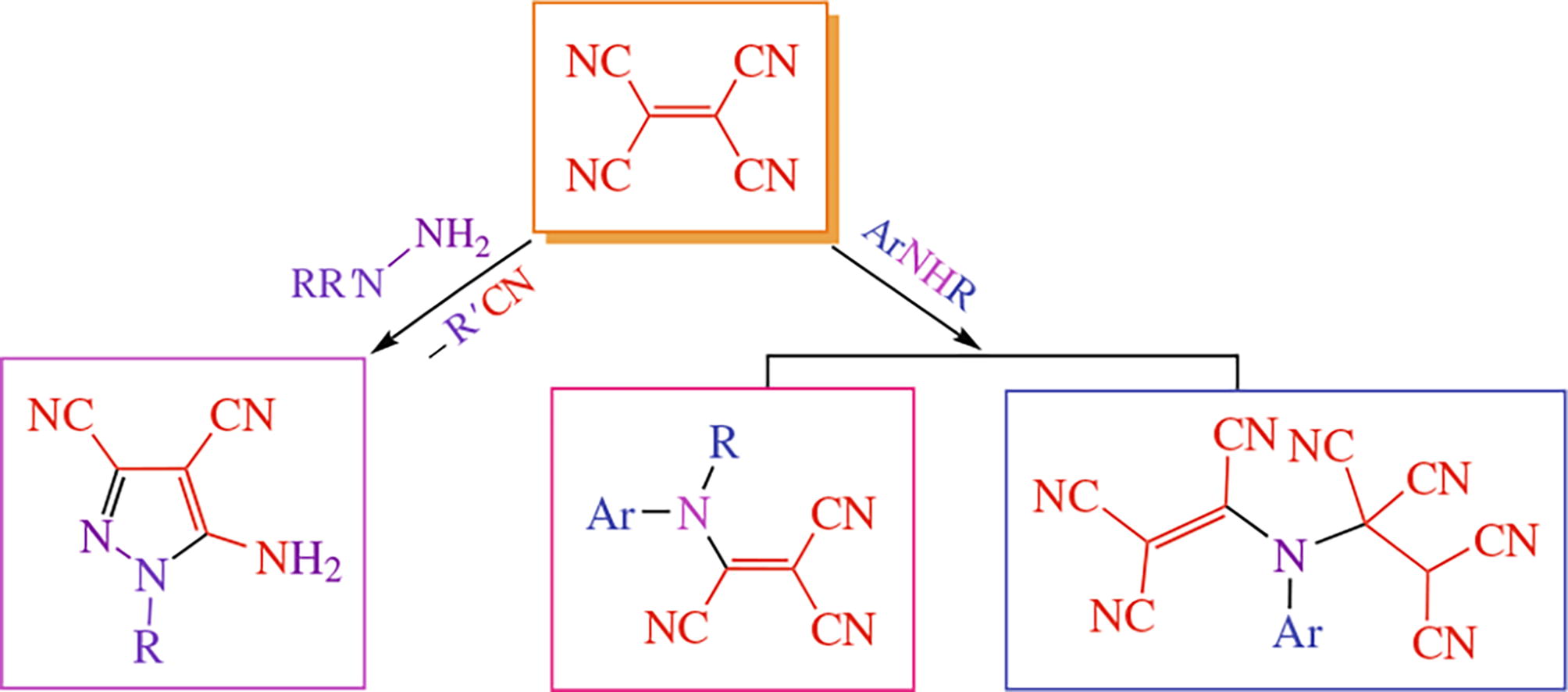
Reaction between tetracyanoethylene and N,N-dimethyl-or N-(2-pyridyl)hydrazines affords 5-amino-3,4-dicyano-pyrazoles. In cases of aromatic amines, replacement of one cyano group by amino one occurs thus giving N-(tricyano-vinyl)anilines.
References
.

Ibata T., Isogami Y., Nakawa H., Tamura H., Suga H., Shi X., Fujieda H.
Bulletin of the Chemical Society of Japan,
1992
.

Fleming F.F., Yao L., Ravikumar P.C., Funk L., Shook B.C.
Journal of Medicinal Chemistry,
2010
.

Belikov M.Y., Ershov O.V., Lipovskaya I.V., Eremkin A.V., Nasakin O.E.
Russian Journal of Organic Chemistry,
2011
.
![Heterocyclic o-Aminonitriles: Preparation of Pyrazolo[3,4-d]-pyrimidines with Modification of the Substituents at the 1- Position](/storage/images/resized/MjH1ITP7lMYGxeqUZfkt2BnVLgjkk413jwBV97XX_small_thumb.webp)
Al-Afaleq E., Abubshait S.
Molecules,
2001
.
![Synthesis and Biological Evaluation of 1-Aryl-4,5-dihydro-1H-pyrazolo[3,4-d]pyrimidin-4-one Inhibitors of Cyclin-Dependent Kinases](/storage/images/resized/iLiQsFqFaSEx6chlGQ5fbAwF6VYU3WWa08hkss0g_small_thumb.webp)
Markwalder J.A., Arnone M.R., Benfield P.A., Boisclair M., Burton C.R., Chang C., Cox S.S., Czerniak P.M., Dean C.L., Doleniak D., Grafstrom R., Harrison B.A., Kaltenbach R.F., Nugiel D.A., Rossi K.A., et. al.
Journal of Medicinal Chemistry,
2004
.
![Synthesis and Reactions of Novel Fused 1-(8-Hydroxy-7-iodoquinoline-5-sulfonyl)-1H-pyrazolo-[3,4-d]pyrimidine-3-carbonitrile Derivatives](/storage/images/resized/oZgeErrVFhuDksyqFURLvYS1wtVSBWczh001igGo_small_thumb.webp)
Khalifa N.M., Eweas A.F., Amr A.E.
Russian Journal of General Chemistry,
2018
.

Belikov M.Y., Ershov O.V.
Russian Journal of Organic Chemistry,
2019
.

Sausen G.N., Engelhardt V.A., Middleton W.J.
Journal of the American Chemical Society,
1958
.

McKusick B.C., Heckert R.E., Cairns T.L., Coffman D.D., Mower H.F.
Journal of the American Chemical Society,
1958
.

Miyashi T., Kamata M., Mukai T.
Journal of the American Chemical Society,
1987
.

Sanghvi Y.S., Bhattacharya B.K., Kini G.D., Matsumoto S.S., Larson S.B., Jolley W.B., Robins R.K., Revankar G.R.
Journal of Medicinal Chemistry,
1990
.

Aly A.A., Gomaa M.A.
Canadian Journal of Chemistry,
2005
.

Wilding B., Winkler M., Petschacher B., Kratzer R., Glieder A., Klempier N.
Advanced Synthesis and Catalysis,
2012
.

Shimizu S., Haseba Y., Yamazaki M., Kumazawa G., Kobayashi N.
Chemistry - A European Journal,
2014
.
![Notiz zum Reaktionsverhalten von Bis‐[β‐chlor‐äthyl]‐tricyanvinyl‐amin im Vergleich zu N.N. ‐Bis‐[β‐chlor‐äthyl]‐ p ‐tricyanvinyl‐anilin](/storage/images/resized/bRyGpdm98BkAUYiK1YFNpl5Z7hPu6Gd87gbIeuG3_small_thumb.webp)
Schulze W., Willitzer H.
Chemische Berichte,
1967
.
![Pyrazolo[3, 4-d]pyrimidines related to lonidamine](/storage/images/resized/bRyGpdm98BkAUYiK1YFNpl5Z7hPu6Gd87gbIeuG3_small_thumb.webp)
Gatta F., Luciani M., Palazzo G.
Journal of Heterocyclic Chemistry,
1989
.
![The reaction of 2-amino- N ′-arylbenzamidines with tetracyanoethene reinvestigated: routes to imidazoles, quinazolines and quinolino[2′,3′:4,5]imidazo[1,2- c ]quinazoline-8-carbonitrile](/storage/images/resized/GDnYOu1UpMMfMMRV6Aqle4H0YLLsraeD9IP9qScG_small_thumb.webp)
Mirallai S.I., Manoli M., Koutentis P.A.
Tetrahedron,
2015
.

Hassan A.A., Mohamed S.K., Abdel-Latif F.F., Mostafa S.M., Mague J.T., Akkurt M., Abdel-Aziz M.
Tetrahedron Letters,
2016
.
![Synthesis and pharmacological assessment of diversely substituted pyrazolo[3,4-b]quinoline, and benzo[b]pyrazolo[4,3-g][1,8]naphthyridine derivatives](/storage/images/resized/GDnYOu1UpMMfMMRV6Aqle4H0YLLsraeD9IP9qScG_small_thumb.webp)
Silva D., Chioua M., Samadi A., Carmo Carreiras M., Jimeno M., Mendes E., Ríos C.D., Romero A., Villarroya M., López M.G., Marco-Contelles J.
European Journal of Medicinal Chemistry,
2011
.

Elbastawesy M.A., Aly A.A., Ramadan M., Elshaier Y.A., Youssif B.G., Brown A.B., El-Din A Abuo-Rahma G.
Bioorganic Chemistry,
2019
.

Bennani F.E., Doudach L., Cherrah Y., Ramli Y., Karrouchi K., Ansar M., Faouzi M.E.
Bioorganic Chemistry,
2020
.
![Synthesis of Some Biologically Active Pyrazolylphthalazine Derivatives and Acyclo-C-nucleosides of 6-(2,4,6-trimethylphenyl)-1,2,4-triazolo[3,4-a]phthalazine](/storage/images/resized/Vb3hw5ha3GXFySkcFIRq7hedUzMGRVNprYvOwnlQ_small_thumb.webp)
El-Shamy I.E., Abdel-Mohsen A.M., Fouda M.M., Al-Deyab S.S., El-Hashash M.A.
Asian Journal of Chemistry,
2014
.

Morgan A., Petousis N., van Lier J.
European Journal of Medicinal Chemistry,
1997
.
Podhradský D., Paulíková H., Imrich J.
Collection of Czechoslovak Chemical Communications,
2010